Implant Equipment & Supplies
-
Manufactured by Embody Inc.based in USA
The TAPESTRY Biointegrative Implant is the first collagen-based implant with a bioengineered micro-architecture and bio-stimulative, highly organized type I collagen chemistry specifically designed for tendon repair. TAPESTRY’s proprietary physio-chemistry demonstrated dense collagenous tissue in-growth. ...
-
Manufactured by ZimVie Inc.based in USA
The T3 Dental Implant is Designed to Deliver Aesthetic Results Through Tissue Preservation. T3 Dental Implants: Built for stability and optimized placement: Contemporary Hybrid Surface - Provided by complex multi-surface topography. Seal Integrity - Provided by a stable and tight implant/abutment interface. ...
-
Manufactured by emka Technologies S.A.Sbased in FRANCE
easyTEL+ is a fully implantable digital system, that transmits physiological data from conscious freely moving laboratory animals. It can be remotely managed and ...
-
Manufactured by Medentika - Institut Straumann AGbased in GERMANY
The model is made with prefabricated, high precision, rotationally stable stainless steel and titanium laboratory implants that are labelled to make them easier to ...
-
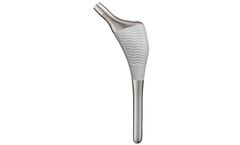
Manufactured by Lape Medicalbased in FRANCE
STEM ; Cementless anatomical stem : TA6V titanium Cemented anatomical stem: 316L stainless steel 11 right and left ...
-
Manufactured by Micropulse, Inc.based in USA
The Micropulse implant team serves the spine, trauma, and upper and lower extremity markets. A focus to automate machining processes has allowed for continued growth and expansion of this product line. A diverse machining foundation, skilled staff, and a mature QMS platform allows Micropulse to strategically grow with an established customer base in an industry known for its ...
-
Manufactured by Xenco Medicalbased in USA
The implants are available in a range of sizes to accommodate variations in patient anatomy. Spacers are manufactured from PEEK polymer and contain tantalum markers to assist with radiographic visualization. The sterile, SETx Hyperlordotic systems may be used in the treatment of Flat Back ...
-
Manufactured by Lape Medicalbased in FRANCE
After having inserted the cage in the inter-somatic space, the blade is turned using a gripper / screwdriver which secures the implant. A primary stability is obtained limiting the possible risks of cage migration before the graft has taken. This securing is reversible allowing the ablation of the implant without risk of deterioration of the vertebral ...
-
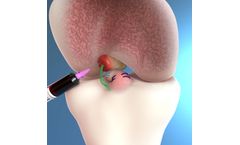
Manufactured by Dentis Co., Ltd.based in SOUTH KOREA
Platform switching helps to minimize bone loss that can reduce peak-stress and thereby preserve marginal bone. Effective to establish a certain biological width of the peri-implant mucosa. ...
-
Manufactured by Lape Medicalbased in FRANCE
The ASPEN cage is the 1st cervical cage placed on the market pre-filled with a bone substitute and equipped with a complete single-use ancillary. Made of PEEK Optima, it has two gold radiological markers and is anatomically shaped. Retentive ridges provide primary ...
-
Manufactured by LZQ Tool Co., Ltd.based in CHINA
Medical tools and parts series(Abutment/Transfer/Analog/Healing cap/screw) (ultra-high precision type & different material and color series). Temporary abutments should be placed with finger pressure only, especially the 2.5mm posts. Temporary abutments are made of surgical grade titanium alloy Ti6Al4V. The hemispherical portion of a temporary abutment matches the profile of the ...
-
Manufactured by Acrotec Medtechbased in SWITZERLAND
Acrotec Medtech companies specialise in the custom production of implant holders, offering secure support tailored to surgical procedures. Designed with durability and ease of use in mind, our holders ensure safe handling of implants, reducing the risk of contamination and ensuring optimal placement during surgery. ...
-
Manufactured by Novastep Inc.based in USA
Clear Confidence for Better Results; Versatile Cannulated Implant system: Headless and Headed Compression Screws. Full Range of Implant Diameters: Available in 2.5, 4.3, 5.6, 7.4mm. Sterile, Disposable ...
-
Manufactured by ARCH Medical Solutions Corp.based in USA
From cervical to lumbar to SI fusion, we develop spinal devices used to treat deformity, stabilize and strengthen the spine while facilitating fusion. Devices can now be optimized to better match the modulus of bone and also provide better surgical visibility through the use of biocompatible titanium. Additive manufacturing is revolutionizing spine surgical outcomes for both surgeons and ...
-
Manufactured by PolyActiva Pty Ltd.based in AUSTRALIA
Being developed to reduce the risk of Endophthalmitis following ocular (cataract) surgery. Clinical Investigation Stage: Phase ...
-
Manufactured by Dentsply Sironabased in USA
Ankylos is an implant system for clinicians who demand excellent results. With more than 30 years’ proven clinical experience, this innovative, worldclass implant technology is thoroughly established and deliver what all patients want – front-row smiles. ...
-
Manufactured by LZQ Tool Co., Ltd.based in CHINA
The constant emergence profile of the different prosthetic components helps to facilitate manipulation during the various prosthetic phases, providing improved predictability of aesthetic outcome and painless insertion, without tissue tension from the components in the ...
-
Manufactured by Miach Orthopaedics, Inc.based in USA
The BEAR Implant is an ideal bridge designed to restore the native ACL. Validated by Level 1 clinical evidence, No harvest site morbidity or need for donor tissue, Easy, reproducible procedure, Indicated for a broad range of ACL tear types. The BEAR Implant is a decellularized, bovine-derived, type I collagen implant that resorbs within 8 weeks ...
-
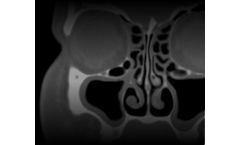
Manufactured by Intersect ENT, Inc.based in USA
Contraindications include patients with intolerance to mometasone furoate (MF) or hypersensitivity to bioabsorbable polymers. Safety and effectiveness of the implant in pregnant or nursing females have not been studied. Risks may include, but are not limited to, pain/pressure, displacement of the implant, possible side effects of intranasal MF, sinusitis, ...
-
Manufactured by CurvaFix, Inc.based in USA
Curved implant for curved bones of the pelvis. The CurvaFix IM Implant is the first, new intramedullary pelvic trauma technology in 30 years, and is the only device capable of following the natural bone shape and filling the space within the pelvis. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you