COVID-19 Test Kit Equipment & Supplies
-
Manufactured by DxTerity Diagnostics Inc.based in USA
Our SARS-CoV-2 RT-PCR saliva-based test is FDA EUA authorized for at home unsupervised sample collection, making easy for you to take your test in the comfort of your home. Collect your sample at home and then ship it back to our lab. We use state-of-the-art technology to get your accurate results. ...
-
Manufactured by Fluxergybased in USA
Learn more about Fluxergy’s qualitative real-time reverse transcription polymerase chain reaction (RT-qPCR) test. For Research Use Only (RUO). Not for use in diagnostic ...
-
Manufactured by Fluxergybased in USA
Potential environments for rapid testing applications include emergency rooms, outpatient procedures, urgent care, workplace screening, mobile testing, community level testing settings, school reopenings, and tourism. Fluxergy’s easy-to-use qualitative RT-qPCR test can detect SARS-CoV-2 in one hour. ...
-
Distributed by Alban Scientific, Inc.based in USA
QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple. The test is authorized for home use with self-collected anterior nasal (nares) swab samples in individuals aged 2 and ...
-
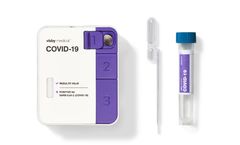
Manufactured by Visby Medical, Inc.based in USA
Fast, accurate, and actionable PCR results in under 30 minutes. Instrument-free PCR: Empower on-demand testing, when and where you need ...
-
Manufactured by LabTurbo Biotech Corporationbased in TAIWAN
LabTurbo™ AIO COVID-19 RNA Testing Kit is a multiplex real-time reverse transcription polymerase chain reaction, (RT-PCR) diagnostic product for SARS-CoV-2 RNA (COVID-19) detection. The multiplex reaction detects COVID-19 N1 gene, coronavirus E gene, and ...
-
Manufactured by Talis Biomedical Corporationbased in USA
A front-line choice for quick, high volume SARS-CoV-2 antigen testing. Testing for COVID-19 is in high demand. SARS-CoV-2 antigen testing can be an alternative first line of defense when compared to PCR testing, particularly for symptomatic patients within a specific window from symptom onset and ...
-
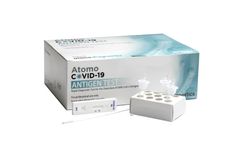
Manufactured by Atomo Diagnostics Limitedbased in AUSTRALIA
A fast, accurate point of care COVID-19 antigen test for the qualitative detection of nucleocapsid protein antigens specific to the SARS-CoV-2 virus. Utilising an anterior nasal swab specimen obtained from individuals suspected of having COVID-19, the Atomo COVID-19 Antigen ...
-
Manufactured by DxTerity Diagnostics Inc.based in USA
The DxTerity SARS-CoV-2 (COVID-19) RT-PCR CE saliva-based test provides the convenience of at-home sample collection with results available within 24-72 hours. Our highly-sensitive, gold-standard PCR test has been verified to work with all COVID-19 ...
-
Manufactured by Biomedal S.L.based in SPAIN
The rapid COVID-19 Antigen test is a qualitative in vitro diagnostic test designed for professional use, which detects the presence of SARS-CoV-2 virus proteins in the sample allowing an early detection of the infection. The test includes all the necessary material for its use in nasopharyngeal ...
-
Manufactured by Spark Mobile Diagnosticsbased in INDIA
CoV-Check DIRECT Covid-19 Antigen Test is a rapid immunochromatographic assay that utilizes specific monoclonal antibodies to detect Nucleocapsid protein of SARS-CoV-2 virus in nasopharyngeal swab specimens. This test provides a quick and easy to use test to help in the diagnosis of SARS-CoV-2 infection to ...
-
Manufactured by SD Biosensorbased in SOUTH KOREA
STANDARD Q COVID-19 Ag Home Test is a rapid chromatographic immunoassay for the qualitative detection of SARS-CoV-2 nucleocapsid antigen present in human nasal ...
-
Distributed by Hardy Diagnosticsbased in USA
An immunochromatographic rapid diagnostic test (RDT) intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct nasopharyngeal (NP) or anteriornasal (AN) swab specimens. Authorized for use under an Emergency Use Authorization (EUA) and for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of ...
-
Manufactured by HiberGene Diagnosticsbased in IRELAND
HiberGene’s PCR Adapt COVID-19 RT-qPCR is a CE marked multi-target SARS-CoV-2 test for nasopharyngeal (NP), oropharyngeal (OP) and anterior nasal swabs. This single tube assay is validated for both direct sample testing and manual/automated extraction. ...
-
Manufactured by Elers Medical Finland Ltd.based in FINLAND
The NADAL® COVID-19 antigen rapid tests deliver highly precise results in just a few minutes, cost a fraction of PCR tests and thus free up laboratory capacities. The NADAL® COVID-19 Antigen Rapid Test – a chromatographic lateral flow immunoassay – checks for ...
-
Manufactured by HymonBio Co.,Ltdbased in CHINA
Hymon COVID-19 antigen rapid test kit is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) with nasal swabs or saliva during the acute phase of infection. It only takes 15 minutes to complete the test and the test is also non-invasive. ...
-
Manufactured by Biomedal S.L.based in SPAIN
The COVID-19 Rapid Test is a qualitative diagnostic test that uses lateral flow immunochromatography technology for the specific detection of human IgG and IgM anti SARS-CoV-2 antibodies and allows the detection of such antibodies in serum, plasma or whole blood samples. ...
-
Manufactured by HTL-Strefa S.A.based in POLAND
Today we are taking the next step creating our portfolio of effective, simple and specific solutions, but we are also thinking about the future. Here our complete line to better face the needs related to ...
-
Manufactured by DxTerity Diagnostics Inc.based in USA
COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. ...
-
Distributed by Labex Canada Inc.based in CANADA
The COVID-19 Vaccine IgG Rapid Test is a lateral flow immunoassay intended for the qualitative detection of antibodies binding to the Receptor Binding Domain (RBD) of the spike protein of the SARS-CoV-2 virus or COVID-19 vaccine in human serum, plasma or whole blood. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you