Bone Regeneration Equipment & Supplies
-
based in FINLAND
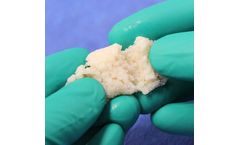
Bonalive granules is a unique bone regeneration technology that naturally inhibits bacterial growth and stimulates bone ...
-
Manufactured by Norakerbased in FRANCE
Inspired by an innovative and reliable technology, Glassbone injectable putty is indicated in orthopedic CMF and spine ...
-
based in USA
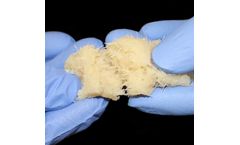
Today there is no satisfactory solution for major bone injuries caused by accidents, warfare and cancer, especially complex and large segmental bone loss. Our NuCress™ bone scaffold technology has been shown to promote healing of large segmental bone breaks (>2.5 cm) tested in large and small animals (goats, mice) ...
-
Manufactured by Aziyo Biologics, Inc.based in USA
OsteGro Allograft Bone Matrix, the second product in this family, is a sterile bone matrix comprised of cancellous particles and demineralized cortical ...
-
Manufactured by Aziyo Biologics, Inc.based in USA
ViBone is comprised of cancellous bone particles with preserved cells and demineralized cortical bone particles produced via Aziyo’s gentle proprietary VBM ...
-
Manufactured by Biovision GmbHbased in GERMANY
LeadFIX is a bioresorbable membrane pin made from polylactide. The clinical use of resorbable membranes for guided bone regeneration constitutes a new approach in dental surgery. LeadFIX is a bioresorbable augmentation system used to fix membranes in position. Total membrane stability is an important pre-requisite for successful, guided bone ...
-
Manufactured by Surgical Solutionsbased in PUERTO RICO
An integrated approach to bone regeneration offering a full portfolio of base allograft, dbm, and bone ...
-
Manufactured by Jeil Medical Corporationbased in SOUTH KOREA
Jeil Tack System for Mesh Fixation in Dental Bone ...
-
Manufactured by Aziyo Biologics, Inc.based in USA
Viable Bone Matrix for Spine and Orthopedic Procedures. Fiber Viable Bone Matrix: Fiber Viable Bone Matrix (VBM) is made of cancellous bone particles with preserved cells combined with demineralized cortical fiber produced via Aziyo’s gentle proprietary VBM ...
-
Manufactured by botiss biomaterials GmbHbased in GERMANY
During the application of modern GBR and GTR techniques, barrier membranes are indispensable to achieve predictable and reliable results. By fixation of the barrier membrane to the local bone, the application of the particulate bone regeneration material as well as the coverage of the augmentation site by the barrier membrane can be significantly ...
-
Manufactured by Bonegraft Biyolojik Malzemeler San. VE TIC. A.S.based in TURKEY
The ergonomic design of the applicator provides easy removal of the pin and easy fixation of the membrane. The notch, which is about half the length of the pin, allows the pin to attach to the bone. The application of particulate bone regeneration material from the barrier membrane to the local bone, as well as the covering of ...
-
Manufactured by Osteogenics Biomedicalbased in USA
Often used with on-lay grafting in ridge augmentation procedures, Cytoplast™ Ti-150 titanium-reinforced membranes provide clinicians with the predictability they are accustomed to with the Cytoplast™ TXT-200 membranes, along with the added benefit of a titanium frame to maintain space during guided bone regeneration. These membranes are also popular ...
-
Manufactured by Osteogenics Biomedicalbased in USA
Often used with on-lay grafting in ridge augmentation procedures, Cytoplast™ Ti-250 titanium-reinforced membranes provide clinicians with the predictability they are accustomed to with the Cytoplast™ TXT-200 membranes, along with the added benefit of a titanium frame to maintain space during guided bone regeneration. These membranes are also popular ...
-
Manufactured by INNOTERE GmbHbased in GERMANY
The final material consists of synthetic calcium phosphates, mainly alpha-tricalcium phosphate and nanocrystalline, calcium deficient hydroxyapatite, which makes the scaffolds ideal substrates for cell culture in the field of bone regeneration. INNOTERE provides services for cement composition modification, including additives, creation of design files, and ...
-
based in SOUTH KOREA
The bone graft material is purified bone mineral derived from bovine (OCS-B) or equine (OCS-H) bone. Also, OCS-B and OCS-H have good biocompatibility and osteoconductivity. OssGen-X15 increases cell attachment (early stage of bone regeneration) and mineralization (last stage of bone ...
-
Manufactured by Nobel Biocarebased in SWITZERLAND
A membrane with outstanding handling1,2 that facilitates bone gain. A resorbable collagen membrane for guided bone regeneration and guided tissue regeneration ...
-
Manufactured by Jeil Medical Corporationbased in SOUTH KOREA
Bone Screws for Fixation of Bone Blocks and Mesh in Dental Guided Bone ...
-
Manufactured by EnColl Corporationbased in USA
Over 100 years of research, experimentation and use has demonstrated that calcium sulphate enhances bone regeneration and is totally biocompatible and and resorbable. At the same token, high purity type-I collagen DMBM (xenograft) also is essential for tissue regeneration and remodeling. Osseomold consists of both materials admixed in proper ...
-
based in FINLAND
Bonalive putty MIS is a smart bone regeneration technology, providing stable positioning and controlled access in minimally invasive ...
-
Manufactured by Medical Device and Implants (MDI)based in USA
Limb lengthening is a reconstructive procedure where the deformed bone is straightened or missing bone is replaced. It is performed in children and adults who have variations in their leg length as a result of diseases, injuries or birth defects. The process of increasing the bone length depends on tissue and bone ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you