Surgery Equipment Supplied In Minnesota
-
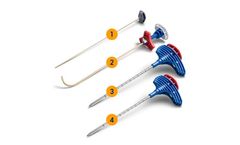
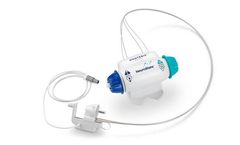
Manufactured by Relievant Medsystems, Inc.based in USA
The Intracept Access Instruments are engineered for precise targeting of the BVN through a traditional unipedicular approach. The procedure is performed under fluoroscopic imaging, similar to many other minimally invasive spine procedures ...
-
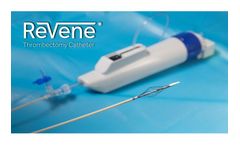
Manufactured by Surmodics, Inc.based in USA
The Vetex Medical ReVene® Thrombectomy Catheter has been engineered with a patented dynamic cage technology that enables the predictable removal of wall adherent thrombus in a single session without the need for ...
-
Manufactured by Shockwave Medical Inc.based in USA
Neovasc is focused on creating better outcomes for difficult-to-treat cardiology patients through the development of two novel products: Reducer™* for treatment of refractory angina and the Tiara™ System* for treatment of mitral valve regurgitation. Millions of patients worldwide with coronary artery disease who are not candidates for revascularization suffer from refractory angina ...
-
based in USA
Different Platforms for Your Individual Needs. NexPak®X Intranasal Splint is intended to prevent adhesions in the nasal and sinus cavity after surgery or trauma while minimizing bleeding and edema. NexPak®X Intranasal Splint is indicated as an intranasal splint intended to minimize bleeding and edema and to prevent adhesions between the septum and the nasal cavity. It is place in the ...
-
Manufactured by EPIEN Medical, Incbased in USA
ORALMEDIC Barrier Solution is a safe therapeutic chemical curettage agent. ORALMEDIC Barrier Solution is the only treatment available that stops oral ulcer pain, seals damaged mucosal tissue, cleans cellular debris and aids the natural healing ...
-
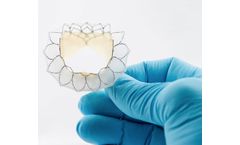
Manufactured by Anteris Technologies Limitedbased in AUSTRALIA
Evidence of Long-Term Durability: 25 patients were followed for up to 10 years; No graft failure, thromboembolic events, infections or device-related reinterventions; Echocardiographic assessment demonstrated the absence of calcification in all ...
-
Manufactured by Spineology Inc.based in USA
The Elite™ Expandable Interbody Fusion System represents the next generation of expandable technology. Its unique design minimizes both neural retraction and insertion force, accommodates a larger graft channel than many competitive expandable cages, and provides controlled expansion to restore disc height, potentially providing indirect decompression. When paired with Incite Cortical ...
-
Manufactured by Spineology Inc.based in USA
Minimally invasive, bone-sparing surgical methods and large load-bearing interbody fusion systems have dramatically reduced the biomechanical demands placed on fixation systems, eliminating the need for large complex spinal fixation constructs for many patients. The Capture Facet Fixation System is the perfect complement. ...
-
Manufactured by Spineology Inc.based in USA
The VIA Spinous Process Fixation System is a next-generation system, utilizing contoured surfaces and independent compression to accommodate varying spinal anatomy. ...
-
Manufactured by MPR Orthopedicsbased in USA
Used to help nurses elevate patients’ hands after surgery. Originally designed by hand surgeon Dr. Peter Carter to help nurses elevate patients’ hands after surgery, Carter Pillows are also helpful for upper limb fracture outpatient care–patients take it home from ER for easy, appropriate ...
-
Manufactured by Sanuwave and Sanuwave Health, Inc.based in USA
PACE® Therapy Offers Patients the Best Chance of Healing: Use Energy First. Diabetic patients that develop chronic wounds have comorbidities that can make healing difficult. They deserve the greatest chance for accelerated wound healing. Our leading, end-to-end, advanced wound care solution, SANUWAVE’s dermaPACE System activates the body’s normal regenerative processes, expedites ...
-
Manufactured by Monteris Medicalbased in USA
The Robotic Probe Driver, designed for use with a skull anchor like the Monteris Mini-Bolt, reduces profile in the MRI for access to virtually any brain location along the ideal ...
-
Manufactured by Monteris Medicalbased in USA
Dependable skull fixation for neurosurgical devices. The only cranial fixation bolt with a robotic interface to enable precise laser delivery. ...
-
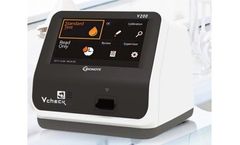
Manufactured by Bionotebased in USA
Bionote's Vcheck V200 analyzer for veterinary fluorescence immunoassay can help point-of-care testing for various biomarkers, in-clinic at smaller practices and clinics. It helps check quantitative measurement of biomarker levels in serum, plasma and whole blood delivered for analysis via one of Vcheck’s 12 biomarker test strips. As a multiple test analyzer, the V200 can be used as a ...
-
Manufactured by Akkerman Inc.based in USA
The GBM 308A/339A Latching Frames allows operators to install up to 30-in. OD (762 mm) pipe from an 8 or 9-ft. (2.4/2.7 m) minimal diameter shaft and features a 10.5-in. (267 mm) stroke ...
-
Manufactured by LP Surgical Fibersbased in USA
LP Surgical Fibers brings superior value and industry leading quality to both our single use and reusable laser fibers. All of our fibers are packaged sterile for a 5-year shelf life and come standard in 272, 365, 550 and 940 micron diameters. Each fiber is terminated with an SMA-905 connector for open platform lasers as well as select manufacturer specific connectors. Our Silica/Silica fibers ...
-
Manufactured by Innovia Medicalbased in USA
One Hole V Type Re-Injection Cannula, 3-C-1HV- diameter x length, Forked end to pre-tunnel injection site. Pretunnel or dissect tissue. Stainless Steel. Autoclavable. Select hub style, length and diameter. For more specific sizes/lengths, please call ...
-
Manufactured by MGC Diagnostics Corporationbased in USA
The GoSpiro® spirometer, an MTI proprietary device and app, is the first spirometer designed for connected health applications and remote patient monitoring. This device provides diagnostic quality test results and delivers spirometry data that is reimbursable and able to be conducted at home or in a clinic. ...
-
Manufactured by Minnetronix Medicalbased in USA
Delivering custom RF technologies, quickly and reliably. Our RF-specific processes incorporate time-tested learnings and best practices to solve the toughest problems from design through manufacturing, without compromising customization or performance. ...
-
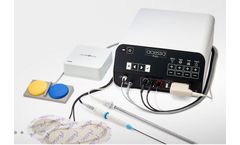
Manufactured by Carbon Medical Technologies, Inc.based in USA
Durasphere is an injectable bulking agent containing pyrolytic carbon-coated graphite beads suspended in a water-based carrier gel containing beta glucan. It is used in the treatment of stress urinary incontinence. Durasphere is a highly biocompatible, permanent material that can be administered in less than 30 minutes in a simple office-based ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you