Surgery Equipment Supplied In Indiana
-
PremiumManufactured by B Medical Systemsbased in LUXEMBOURG
Plasma Contact Shock Freezer: Set Temperature: - 50°C. Climate class (ambient temperature range): +10°C to +32°C. External dimensions (HxWxD): 1910 x 1080 x 830 mm | 75.20 x 42.52 x 32.68 in. Refrigerant Type: R449a. ...
-
PremiumManufactured by B Medical Systemsbased in LUXEMBOURG
Plasma Contact Shock Freezer: Set Temperature: - 50°C. Climate class (ambient temperature range): +10°C to +32°C. External dimensions (HxWxD): 1910 x 1500 x 830 mm | 75.20 x 59.06 x 32.68 in. Refrigerant Type: R449a. ...
-
PremiumManufactured by B Medical Systemsbased in LUXEMBOURG
Plasma Contact Shock Freezer: Set Temperature: - 50°C. Climate class (ambient temperature range): +10°C to +32°C. External dimensions (HxWxD): 1910 x 1500 x 830 mm | 75.20 x 59.06 x 32.68 in. Refrigerant Type: R449a. ...
-
PremiumManufactured by B Medical Systemsbased in LUXEMBOURG
Plasma Contact Shock Freezer: Set Temperature: - 50°C. Climate class (ambient temperature range): +10°C to +32°C. External dimensions (HxWxD): 1910 x 1080 x 830 mm | 75.20 x 42.52 x 32.68 in. Refrigerant Type: R449a. ...
-
PremiumManufactured by B Medical Systemsbased in LUXEMBOURG
Set Temperature: - 50°C. Climate class (ambient temperature range): +10°C to +32°C. External dimensions (HxWxD): 1910 x 1080 x 830 mm | 75.20 x 42.52 x 32.68 in. Refrigerant Type: R449a. ...
-
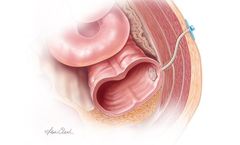
Manufactured by Cook Biotech Inc.based in USA
The Biodesign Enterocutaneous Fistula Plug is for implantation to reinforce soft tissue for repair of enterocutaneous ...
-
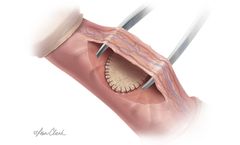
Manufactured by Cook Biotech Inc.based in USA
The Biodesign Peyronie’s Repair Graft is intended for implantation to reinforce soft tissue where weakness exists in the urological anatomy, including but not limited to repair of tunica albuginea defects and reinforcement in the repair of Peyronie’s ...
-
Manufactured by RELIGN Corporationbased in USA
RELIGN’s Tricera™ System is an innovative, integrated arthroscopic radiofrequency device, shaver, bone cutter, and fluid management system. The intuitive touchscreen display provides a quick and user-friendly setup. RELIGN’s integrated technology consolidates multiple consoles into one, minimizing space, setup, and maintenance of multiple ...
-
Manufactured by RELIGN Corporationbased in USA
Exoflow™ is RELIGN’s outflow-only fluid management offering that provides surgeons the flexibility to use their existing inflow fluid management setup while automating outflow through RELIGN devices. Exoflow features a “lock and load” self-loading cassette. The outflow activates automatically with device use as well as on-demand when the flush mode is ...
-
Manufactured by DeRoyal Industries, Inc.based in USA
Easy to use telescopic fold with cuff. Clear, elasticized fenestration for secure fit around endoscope. Optional adhesive tape strip and rubber bands to further secure drape closure. Stretchable neck material ensures tight fit. Sterile, Not made with natural rubber ...
-
Manufactured by OrthoPediatrics Corp.based in USA
Sub-laminar polyester band system that provides a fixation option where anatomy or bone quality preclude pedicle screws. Implants are pre-assembled and ready-to-use. Dual-lead implant option provides ability to pass two bands at once, offering both efficiency and enhanced safety. Implants accept 5.5mm and 6.0mm rod diameters in Cobalt Chrome and Titanium. Implants utilize RESPONSE 5.5/6.0mm ...
-
Manufactured by OrthoPediatrics Corp.based in USA
OrthoPediatrics is pleased to launch the next generation Cannulated Screw System! The new system is available in multiple screw diameters including: 2.5mm, 3.0mm, 3.5mm, 4.0mm, 4.5mm and 5.5mm. For each configuration, all screws and instruments are housed in one case/tray – giving the added convenience of an “all-in-one” set. The upgraded instrumentation, and color-coding aid in ...
-
Manufactured by OrthoPediatrics Corp.based in USA
The OrthoPediatrics Wrist Fusion System is an extension of the PediFrag system, and is indicated for wrist arthrodesis and fractures of other small bones in children, adolescents, and small stature adults. The system consists of two implant length offerings, to fit patients both with and without a proximal row carpectomy (PRC). Bending templates are included in the set to assist with ...
-
Manufactured by OrthoPediatrics Corp.based in USA
The ORTHEX Large Bone system is a comprehensive external fixator platform that can be used as a static frame, a quick reduction trauma frame, or a computer-assisted hexapod. This product may not be available in all markets. Please consult your local OP representative for ...
-
Manufactured by Med-Mizer, Inc.based in USA
Activecare ™ bed's fully rotational and adjustable design makes getting in and out of bed easier and provides customized home-like comfort for patients of all mobility levels. Because There's Nothing Like Coming Home: Getting in and out of bed doesn't have to be a challenge. With the ActiveCare ™ Beds and their automated SafeTurn system, it's easy to reposition, stand up, and get out. ...
-
Manufactured by Nexxt Spine, LLCbased in USA
The Blade® Anterior Cervical Plate System is intended for anterior Screw fixation during the development of cervical spine fusion. The spinal fixation device includes Self-Drilling/Self-Tapping Screws, patented ‘Hurricane’ locking mechanism, and pre-contoured 1-5 level plates. Various instruments are also available as part of the Blade® Anterior Cervical Plate System for use ...
-
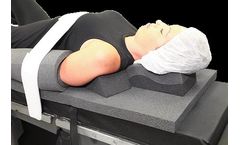
Manufactured by DeRoyal Industries, Inc.based in USA
The STEEP-T™ system provides patient fixation for any procedure that requires steep angulation of the operative table. The system is a solution for multi-specialty surgical applications. The system helps to prevent unwanted movement or slippage of the patient during table position changes as well as during prolonged periods of fixed steep ...
-
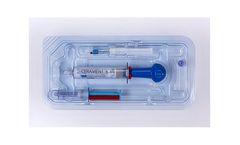
Manufactured by OrthoPediatrics Corp.based in USA
CERAMENT BONE VOID FILLER is an injectable, moldable and drillable synthetic bone void filler consisting of 40% hydroxyapatite, 60% calcium sulfate and the radio-contrast agent iohexol. The unique ratio of hydroxyapatite and calcium sulfate is designed to enable CERAMENT to resorb at the same rate that bone forms. Calcium sulfate acts as a resorbable carrier for hydroxyapatite, and hydroxyapatite ...
-
Manufactured by Valbruna Slater Stainless Inc.based in USA
BIOVAL® AU188Z is the most popular austenitic free machining stainless steel and is the one of most machinable of all the Chromium-Nickel austenitic grades. It’s widely used in applications where the machinability is the most important factor of choice in terms of cost-saving in the production of pieces using multi-spindles and screw ...
-
Manufactured by Zimmer Biometbased in USA
Bonus Synthetic Bone Graft Substitute granules are resorbable, osteoconductive matrices consisting of a thin, 2–10 micron layer of hydroxyapatite over a calcium carbonate core. Bonus Synthetic Bone Graft Substitute has been indicated as a bone graft substitute that resorbs and is replaced with bone during the healing ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you