Preclinical Trial Services
-
Manufactured by ImmunoPrecise Antibodies Ltd.based in CANADA
It enhances our capacity for single-source efficiencies and as an “end-to-end” service provider— from target evaluation through preclinical ...
-
Manufactured by Sartorius AGbased in GERMANY
Similarly, our state-of-the-art technology centre can accommodate development and production of bespoke recombinant albumin suitable for use in discovery through to preclinical ...
-
Manufactured by LVD Biotechbased in SPAIN
Capability to test our manufactured products in vivo in swine ...
-
Manufactured by ARMI - Advanced Regenerative Manufacturing Institutebased in USA
We offer regulatory consulting services to support our member organizations with preclinical study design, FDA submissions and interactions and clinical trial design to support existing industries and grow new ones. ...
-
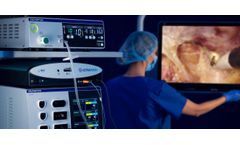
Manufactured by Alesi Surgical Ltdbased in UNITED KINGDOM
Alesi Surgical and WIMAT are co-located and collaborate on research and development, testing, product design, pre-clinical evaluation and post-market clinical follow-up. This provides a unique level of access to experienced surgeons, surgical trainers and trainees, and ...
-
by Oncodesignbased in FRANCE
It covers an extensive spectrum of pharmaceutical expertise and the ability to design and carry out precise and ad hoc assessments in the discovery and development of new therapies.Drawing on our experience and expertise in the preclinical investigation of new therapeutic entities – with different mechanisms of action – in various indications, and given our mastery of ...
-
Manufactured by Creative Bioarraybased in USA
To overcome the weaknesses and limitations of current cell-based and animal-based testing, 3D models with the appropriate biophysical tissue architecture are generated for cardio-toxicity test in order to provide highly predictive and detailed information for the human in vivo study. Creative Bioarray offers the opportunity for highly predictable pre-clinical cardio-toxicity testing to help our ...
-
by Solvias AGbased in SWITZERLAND
Solvias is a highly experienced resource for your chemical development needs, from pre-clinical support and process research and development to kg-scale synthesis. When it comes to chemical development and manufacturing, you can rely on our proven expertise ...
-
based in AUSTRIA
The objective of the Hepatitis B virus (HBV) Program is to utilize the HOOKIPA Technologies to design arenavirus vectors (Lymphocytic Choriomeningitis Virus or Pichinde Virus based) suitable for treatment, cure or prevention of HBV. Together with our partner Gilead Sciences we intend to develop functional therapies for patients already infected with ...
-
Manufactured by Alfa Chemistrybased in USA
Our experimental studies strictly follow ICH, CFDA, FDA and OECD guidelines, providing our clients with high quality data and fast study cycles for preclinical safety evaluation of drugs. alfa chemistry helps our clients to shorten the experimental time and reduce the cost of new drug development through cost control and efficient ...
-
based in USA
Champions Oncology has created screens that enroll each month in either In vivo, Ex Vivo or In vitro platforms. These screens allow for a significant cost savings compared to running stand-alone studies as well as provide free access to Standard of Care response data and other characterization data for each model. Check back frequently to learn more about each screen and to enroll in any active ...
-
Manufactured by Medstone Holding BVbased in NETHERLANDS
Scientific knowledge, distilled and delivered. We combine artificial intelligence and a multi-disciplinary research team to get insights across all stages of development. From early drug discovery and pre-clinical studies to post-marketing surveillance. ...
-
by Solvias AGbased in SWITZERLAND
Our more than 50 R&D experts can rapidly develop scalable, robust and cost-effective chemical processes and optimize reaction conditions for your complex, often chiral, intermediates or APIs. Our processes can be readily transferred to our kilo lab in the form of a proof of concept study, or for scale-up and delivery of material to support your pre-clinical studies. We can also enable you to ...
-
based in ITALY
The Pharmacovigilance system collects and studies adverse events (=noxious) which may be associated to a drug treatment, in order to assess their incidence. identify the cause-effect relationship with the use of the drug. identify any possible risk factors for the occurrence of such adverse events. identify possible means for the prevention of such adverse ...
-
Manufactured by Creative Biolabsbased in USA
Rodent disease models are widely used for a broad range of project objectives, which involve investigation of pathological process and mechanism of disease, pre-clinical tests of potential therapeutic agents, ...
-
Manufactured by Creative Biolabsbased in USA
Prior to clinical trials, it is necessary to conduct animal toxicity studies to best characterize the potential toxicity of new drug candidates. Generally, the objective of preclinical safety evaluation is to determine the toxic effects with respect to target organs, dose dependency, the relationship between clinical findings and exposure to the compound, and ...
-
Manufactured by Sartorius AGbased in GERMANY
A more seamless transition into the clinic. Complex biopharmaceuticals and advanced therapies can present challenges during preclinical development. When implementing an albumin-based solution, Albumedix can help avoid unnecessary complications in your preclinical testing by creating species-specific recombinant albumins to suit your preclinical ...
-
Manufactured by Creative Biolabsbased in USA
In vivo PK studies are critical to ensure drug candidates have appropriate PK properties that can be evaluated in preclinical pharmacology and safety studies. In vivo PK studies provide required and useful information that indicates human equivalent doses (HED), no effect levels (NOEL), and pharmacokinetic/pharmacodynamic (PK/PD) drivers. Conducting PK studies also allows the ...
-
Manufactured by Jubilant Therapeutics Inc.based in USA
Agile and flexible to accelerate value creation. Jubilant Therapeutics is open for partnering or strategic. collaborations with biotech, pharma, academia, research labs for: Accelerating clinical development and market launch of our ongoing programs. Discovery/development of new programs in synergistic areas. Conducting research with our drug candidates either as monotherapy or in combination to ...
-
based in GERMANY
Using our platform, we have uncovered novel therapeutic RNAi approaches that aid heart cell regeneration and proliferation. Patents, publications in progress; compounds validated in preclinical ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you