Vascade - Model MVP - Venous Vascular Closure System
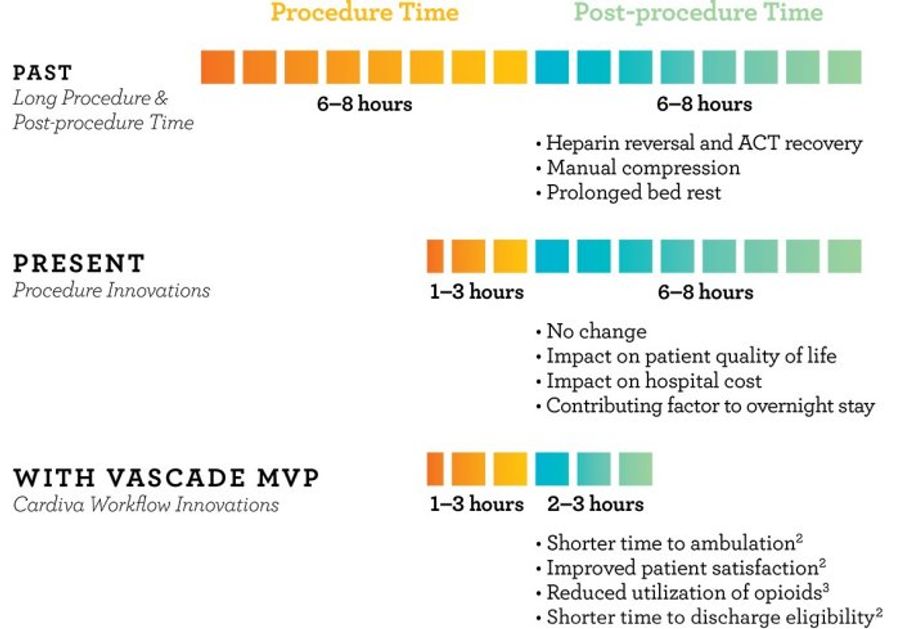
VASCADE MVP eliminates the need for manual compression and reduces time to ambulation by 64%. Get patients up and moving hours earlier, with significantly less discomfort. The only FDA-approved closure device for use following cardiac ablations.
-
Most popular related searches
Simple
Dedicated to mid-bore (6-12F I.D.) multiaccess venous closure
Simple and easy to use
- Single operator
- No sutures or material left in the vessel
Extravascular design
- No permanent or intraluminal implants
Bioabsorbable and thrombogenic collagen plug
- Expands to fill tissue tract
Customer reviews
No reviews were found for Vascade - Model MVP - Venous Vascular Closure System. Be the first to review!