

- Home
- Companies
- Senhance, Brand of Asensus Surgical, ...
- Products
- Senhance - Surgical System
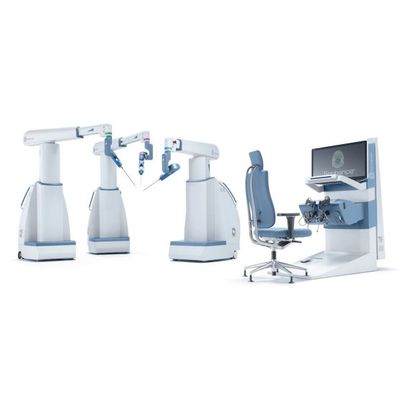
Senhance - Surgical System
The Senhance Surgical System removes the economic limitations of current robotic systems with standard reusable instruments and an open-platform architecture strategy that enables hospitals to leverage existing technology investments. That keeps time and cost-per-procedure comparable to manual laparoscopy, so that the benefits of robotics can reach more patients, in more care settings, for a greater variety of procedures.
Features
The Senhance Surgical System is the driving force behind greater control in laparoscopy
OR Efficiency
The Senhance Surgical System drives greater efficiency in the operating room
Addresses Surgical Variability
The Senhance Surgical System is the driving force behind greater control in reducing variability
Workforce Solutions
The Senhance Surgical System is the driving force to solutions for workforce challenges
US Indication:
The Senhance Surgical System is intended to assist in the accurate control of laparoscopic instruments for visualization and endoscopic manipulation of tissue including grasping, cutting, blunt and sharp dissection, approximation, ligation, electrocautery, suturing, mobilization and retraction. The Senhance Surgical System is intended to be used in general laparoscopic surgical procedures and laparoscopic gynecological surgery. The system is indicated for adult use. It is intended for use by trained physicians in an operating room environment in accordance with the Instructions for Use. The device is restricted to sale by or on the order of a physician.
EU Indication:
The Senhance Surgical System is intended to assist in the accurate control of laparoscopic instruments for visualization and endoscopic manipulation of tissue including grasping, cutting, blunt and sharp dissection, approximation, ligation, electrosurgery, suturing, mobilization, retraction, and sealing of vessels up to and including 5 mm in diameter in laparoscopic and thoracoscopic surgery. The system is indicated for adult and pediatric use. It is intended for use by trained physicians in an operating room environment in accordance with the Instructions for Use. Use of the device is limited to patients with a weight equal to or above 10 kg, who are suitable to be subjected to a conventional endoscopic technique.
Unless otherwise indicated, the legal manufacturer for CE purposes is Asensus Surgical Italia Srl (Notified Body identification number for Class IIa and IIb devices:1936).
The legal manufacturer for CE purposes of bipolar instruments is Günter Bissinger Medizintechnik GmbH.
Eye trackers supplied by Tobii.
The Senhance System was developed under a license of the European Commission Joint Research Centre.
Japan Indication:
The Senhance Surgical System is intended to assist in the accurate control of laparoscopic instruments for visualization and endoscopic manipulation of tissue including grasping, cutting, blunt and sharp dissection, approximation, ligation, electrosurgery, suturing, mobilization, and retraction in laparoscopic surgery. The system is intended for use by trained physicians in an operating room environment in accordance with the Instructions for Use.
Four manipulator arm configurations are only available in CE Markets and Japan.
