

- Home
- Companies
- LKC Technologies, Inc.
- Products
- RETeval - FDA Cleared Non-Mydriatic ...
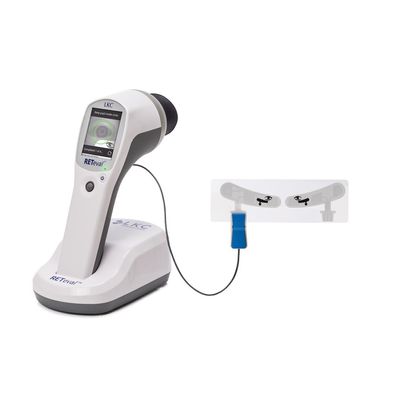
RETeval - FDA Cleared Non-Mydriatic Flash and Flicker ERG/VEP Device
The RETeval device is opening up access to functional information through visual electrophysiology testing in a whole new way. The device is the first completely portable, handheld, full field flash ERG and VEP testing device for medical professionals. Get the full picture of patient eye health with non-invasive, effective and reliable testing – leading to better patient care and outcomes.
Perfect for pediatric patients and adults alike, the RETeval device offers valuable information for a more effective diagnosis and monitoring of vision-threatening diseases. Integrated age-adjusted normative data (reference intervals) provide valuable input to physicians to aid in the interpretation and assessment of patient test results.
LKC’s trusted technology is ISCEV-compliant, FDA cleared, and CE marked. The device features an exclusive DR protocol to make testing for diabetic retinopathy fast, easy, and effective. See more on the RETeval Flicker here.
- Integrated age-adjusted reference data.
- User interface available in 14 languages.
- Designed for use with LKC’s Sensor Strip electrodes for patient comfort.
- Recalibrates light levels at the beginning of every test to ensure long-term consistency of results.
- Non-mydriatic testing by adjusting light levels based on pupil size with real-time pupillography.
- Access to the raw measurements as well as processed data.
- Customize protocols with sinusoidal, square, ramp, and brief-flash stimuli.
- RFF Extractor tool for exporting cursor values, electrical and pupil waveforms and more.
- In-lane testing doesn’t require dedicated space in your space constraint facility.
- Immediate results on the device or in PDF report.
- Pediatric testing without sedation.
