Oxitone - Model 1000M - FDA-cleared Wrist-Sensor Pulse Oximetry Monitor
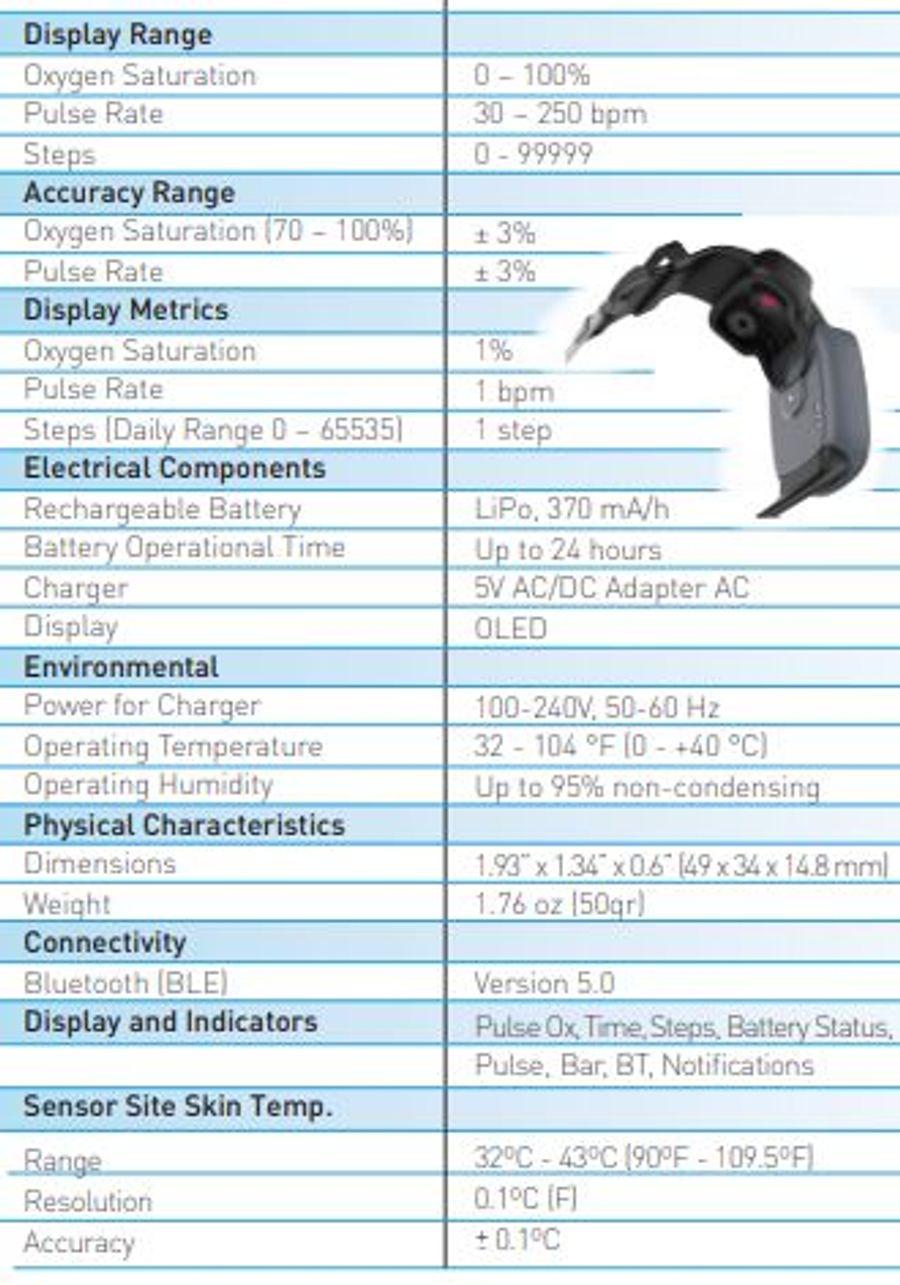
Oxitone 1000M - World’s First FDA-cleared Wrist-Sensor Pulse Oximetry Monitor; No fingertip probe, Bluetooth, Rechargeable long life battery., Wearable, easy to use, FDA-cleared, CE Mark certified.
Details
Medical Follow-up Made Effortless
Follow-up of chronic patients is a costly challenge. Oxitone® has developed the first FDA-cleared wrist-sensor pulse oximetry monitor without fingertip probe. Using real time continuous patient data generated by Oxitone 1000M, clinicians can unlock patients' intelligent insights and effortlessly help thousands of high-risk patients, just in one click.
For high-risk chronic disease patients who need to continue hospital grade monitoring at home
- Blood Oxygen Saturation
- Skin Temperature
- Pulse Rate & Variability
- Fall Detection*
- Steps & Motion
- Time
- Respiratory Rate*
The Ulnar Bone FlexSense™ Technology
Unlike conventional pulse oximeters, Oxitone 1000M is the first FDA-cleared wrist-sensor health monitor that works without a bulky fingertip probe. Depending on the sensor location there are two measurement modes: reflected (back scattered light) and transmitted (forward scattered light). However, in our case the sensor is located at the top of the wrist ulnar bone. We developed a unique flexible sensor that utilizes so called trans-illumination optical technology that enables to copy and follow topography of the wrist. Such sensor configuration and location, resulting from our thorough scientific research followed by intensive clinical validation, represent Oxitone’s technological breakthrough in wrist PPG measurements. The technology is covered by 4 granted US patents.
The device has demonstrated superior accuracy and usability through the data derived for both FDA and CE clearance and numerous clinical pilots.
Customer reviews
No reviews were found for Oxitone - Model 1000M - FDA-cleared Wrist-Sensor Pulse Oximetry Monitor. Be the first to review!