Lupagen Side CAR-T - Delivery System for Cell Therapy and Safety & Clinical
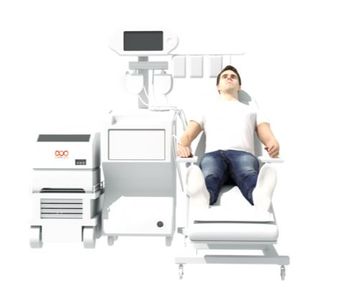
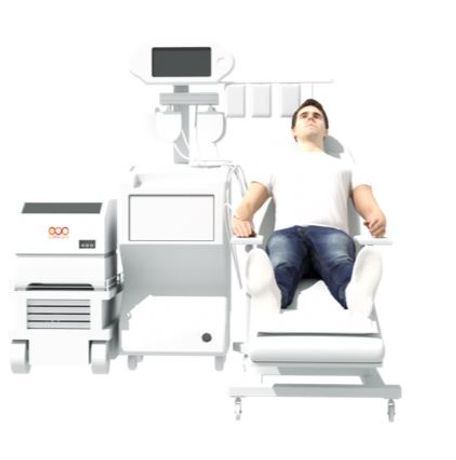
Lupagen avoids the cost & complexity of cell therapy and the safety & clinical challenges of viral-based gene therapy by a simple patient-connected gene delivery procedure done at the bedside. Our Side CAR-T® delivery system, a first-in-class bedside system enables gene delivery directly to target cells in a highly-controlled and safe environment before reinfusing therapies to the patient in a simple out-patient procedure, resulting in significantly lower treatment burden and cost.
Product Details
Nanoparticle platforms, widely used to deliver RNA and DNA, can be delivered elegantly to targeted peripheral blood mononuclear cell populations to generate cell & gene therapies addressing oncology, respiratory/allergy, autoimmune and rare disease indications.
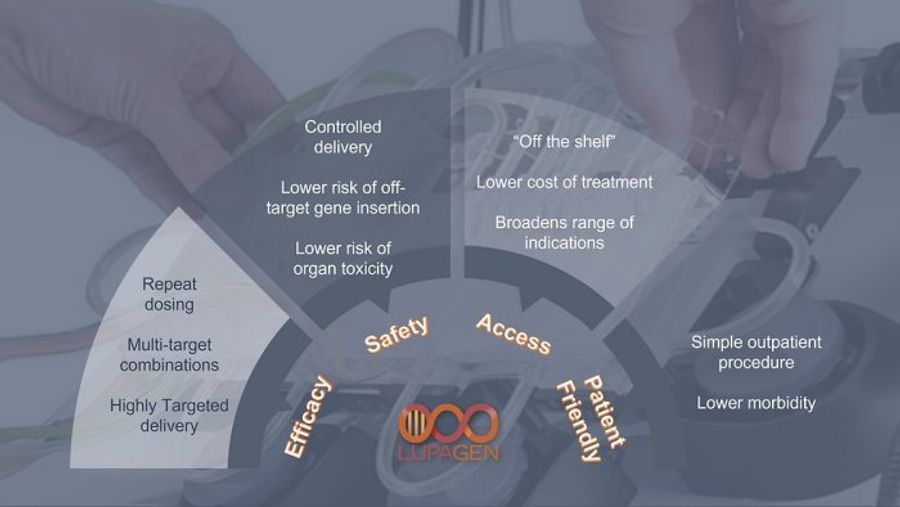
Lupagen’s flexible platform agnostic development approach combined with our proprietary bedside delivery system results in:
- Broader array of targets and indications that can be developed
- Ability to leverage a wide range of nanoparticles systems (lipid, polymer, coated virus)
- SAFER and MORE EFFECTIVE targeted delivery of nanoparticles than can be achieved in-vivo by avoiding current systemic toxicity and dosing challenges
Platform Benefits
Cost effective cell & gene therapy production and safety remain major challenges to expanded use and adoption. Numerous efforts are underway to solve these issues, including decentralized point of care cell processing, use of allogeneic cells, and in-vivo viral gene delivery, but they are all greatly limited in cost savings or face significant efficacy, technical and safety hurdles.
By contrast, Lupagen’s extracorporeal in-vivo gene therapy platform can deliver the best elements of autologous cell therapy and highly scalable biologics without the safety challenges of in-vivo viral gene therapy.
This will lead to multiple benefit across the heal...
- This will lead to multiple benefit across the healthcare ecosystem which include:
- Improved safety
- Broadened addressable treatment populations and indications
- Reduced regulatory burden
- Avoidance of capital and labor costs for centralized or decentralized GMP manufacturing
- Radically lowered cost of goods
- Convenient repeat dosing & improved efficacy potential
Customer reviews
No reviews were found for Lupagen Side CAR-T - Delivery System for Cell Therapy and Safety & Clinical. Be the first to review!