Medical Equipment Supplied In Pennsylvania
-
PremiumManufactured by Myron L Companybased in USA
The DIGITAL DIALYSATE METER is specifically designed to test both acetate and bicarbonate dialysate quality. Measure Conductivity, pH and Temperature to ensure proper mixing during dialysate preparation and as a final check of dialysate quality before hemodialysis treatment. There are no strips or reagents that expire or subjective interpretation of results. Internal sensors require the user to ...
-
Manufactured by Tyber Medical LLCbased in USA
High-quality stainless steel cannulated drills. Drill range from Ø2.0 to Ø9.0mm. Cannulation range from Ø0.7 to Ø2.5. Multiple Connection Options. AO quick connect. ¼” Square. Jacobs ...
-
Manufactured by Tyber Medical LLCbased in USA
The NiTy Staple was designed for ease of use and intraoperative ...
-
based in USA
QTORIN™ rapamycin 3.9% is a novel targeted topical therapy being studied for Microcystic Lymphatic Malformations (Micro LM). Palvella is enrolling a phase 2 clinical study for people with Micro ...
-
Manufactured by Medical Device and Implants (MDI)based in USA
MDI will make trial spacers and instrumentation to compliment the range of orthopedic implants we manufacture for our customers. These are often made from stainless steel and require heat treatment, chrome/hard coating and enamel color banding to designate ...
-
Manufactured by Honeywell Safety Productsbased in USA
Body Harnesses meet or exceed all OSHA, ANSI, and CSA requirements and use non-stretching 13⁄4" polyester webbing for sure support. Standard features include friction shoulder straps, sub-pelvic strap, back D-ring, mating buckle chest and leg straps, and pull-free lanyard rings. 400 lb capacity. ...
-
Manufactured by BriaCell Therapeutics Corp.based in CANADA
Developed and characterized by a team of dedicated scientists and clinicians, Bria-IMT™ (SV-BR-1-GM) is a targeted immunotherapy being developed for the treatment of advanced breast cancer (i.e. breast cancer that has spread from the breast or armpit to other areas of the body including the bones, lungs, liver or sometimes the brain). Bria-IMT™ is a genetically engineered human breast ...
-
Manufactured by BriaCell Therapeutics Corp.based in CANADA
Bria-IMT™ (developed from a breast cancer cell line) is a patented (USPTO) immunotherapy approach that directly stimulates the body’s own cancer fighting cells to attack and destroy breast cancer tumors. ...
-
Manufactured by Starr Life Sciences Corp.based in USA
The MouseOx Plus has multiple sensors available to monitor or record measurements on both conscious and anesthetized mice, rats and other small animals.?Record & Monitor Both Anesthetized & Conscious Subjects, Choose Best Sensor Location According To Application, MRI Compatible Senor Available, Obtain Several Physiological Parameters With A Single ...
-
based in USA
Coronavirus surveillance testing for population-level occurrence. For universities, businesses, and communities eager to reconnect and resume everyday activities, surveillance testing of the SARS-CoV-2 virus is essential to helping monitor population level occurrence. Our PCR testing research solution enables comprehensive, high-frequency surveillance testing at a scale to support your entire ...
-
Manufactured by BTG International Inc.based in USA
DIGIFab neutralizes free digoxin within minutes to resolve the cardiotoxic effects and other clinical manifestations of potentially life-threatening digoxin ...
-
Manufactured by Context Therapeutics Inc.based in USA
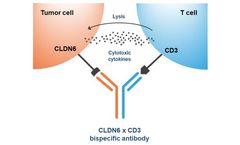
There is growing interest in applying antibody modalities including bispecifics, antibody-drug conjugates, and CART to solid tumors. However, identifying appropriate tumor-specific targets that avoid adverse effects in healthy tissue has been challenging. The tight junction protein Claudin 6 (CLDN6) is a validated therapeutic target for many solid tumor types, including ovarian, endometrial, ...
-
Manufactured by VascuTech Medical, LLCbased in USA
Hemostasis valves are used to help eliminate the backflow of blood during vascular procedures. VascuTech Medical is a leading contract manufacturer of technically sophisticated hemostasis valves and y-adapters including high pressure hemostasis valves utilizing 3-way ...
-
Manufactured by Thrombolex, Inc.based in USA
The BASHIR N-X Endovascular Catheter (BASHIR™ N-X) is a device intended for the localized infusion of physician specified fluids, into the peripheral vasculature, including the pulmonary arteries. ...
-
Manufactured by ImCare Biotech, LLCbased in USA
ImCare Biotech is developing a diagnostic kit, Seravue®, for the early detection of liver cancer, which is supported by an SBIR phase II grant from the National Cancer Institute (NCI, ...
-
Manufactured by VascuTech Medical, LLCbased in USA
Balloon Angiography Catheters are used to widen a narrow or obstructed blood vessel (atherosclerosis) and typically performed by an interventional cardiologist using invasive catheter-based procedures. VascuTech Medical is a leading contract manufacturer of balloon angiography catheters designed to reduce the risk of myocardium contact induced arrhythmias or staining from dye ...
-
Manufactured by NewAge Industries Inc.based in USA
SILCON MED-X tubing is made under strict accordance with Good Manufacturing Procedures and conforms to the biocompatibility requirements of USP Class VI. SILCON MED-X’s surface properties resist sticking and encrustation, and it will not support bacteria growth. The tubing is soft, pliable, and contains no plasticizers which could leach out, causing flow contamination or tube hardening. ...
-
Manufactured by FrenchCreek Production, Inc.based in USA
The 500 series is a long time favorite for maintenance and light construction. Standard features include: Adjustable shoulder, chest, waist and leg straps. Integral 1-3/4″ waist belt. Sliding back D-ring. Lightweight high strength synthetic webbing. Available in sizes: XS, S, M, L, XL, 2XL, 3XL. Full Body Harness, tongue buckle/grommet legs, sewn on 1 3/4″ wide belt, single back ...
-
Manufactured by Cabaletta Bio, Inc.based in USA
Precision Approach to Address the Underlying Cause of Disease: Our second candidate, MuSK-CAART, is designed to treat myasthenia gravis (MG), an autoimmune disease affecting the neuromuscular junction that can lead to motor impairment, muscle weakness, and respiratory ...
-
Manufactured by Synergy Biomedical, LLCbased in USA
BioSphere Flex is a bone graft that was specifically developed to maximize the bone healing potential of bioactive glass. Using Synergy’s proprietary BioSphere Technology, BioSphere Flex is composed of innovative bioactive glass granules combined with a porous collagen/sodium hyaluronate ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you