Medical Equipment Supplied In Ohio
-
PremiumManufactured by Myron L Companybased in USA
The DIGITAL DIALYSATE METER is specifically designed to test both acetate and bicarbonate dialysate quality. Measure Conductivity, pH and Temperature to ensure proper mixing during dialysate preparation and as a final check of dialysate quality before hemodialysis treatment. There are no strips or reagents that expire or subjective interpretation of results. Internal sensors require the user to ...
-
based in USA
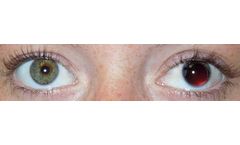
Using pH to confirm NG/OG tube placement is Evidenced Based Best Practice pH is the emerging US/Global Standard for confirming NG/OG tube gastric placement in the NICU. 2005 and 2010, 45% of all cases of harm caused by a misplaced NGT reported by the United Kingdom’s National Patient Safety Agency were due to misinterpreted ...
-
Manufactured by Bryan Medicalbased in USA
TRACOE silcosoft Tracheostomy Tube for neonates and infants, made from silicone. Including: ring obturator, neck strap and disconnection ...
-
Manufactured by Bryan Medicalbased in USA
The smooth outer sheath and laser resistant design of Tenax defines a new standard for ETT. Available in 6 sizes, all Tenax® styles are with ...
-
Manufactured by Bryan Medicalbased in USA
Infinity Esophageal Dilation System is designed for the treatment of swallowing impairment caused by upper esophageal sphincter dysfunction. Infinity contoured to the specific physiology of the upper esophageal sphincter, allowing dilation to nearly double the inflation dimension of conventional ...
-
Manufactured by GOJO Industries, Inc.based in USA
Durable, non-linting wipes; Kills more than 99.99% of most common germs that may cause illness. Removes light soils and dirt from hands. Alcohol-free ...
-
Manufactured by Control Bionics, Inc.based in USA
The NeuroNode Trilogy combines touch, eye control, and EMG/Spatial control for a faster and less fatiguing dedicated speech generating device. The NeuroNode Trilogy has three access methods to provide a flexible communication solution for those with complex speech and physical needs. Multimodal access makes the user more efficient in communicating, up to 47% faster than traditional eye ...
-
Manufactured by VEO Ophthalmics, LLCbased in USA
The CUSTOMFLEX® ARTIFICIALIRIS is intended for use as an iris prosthesis for the treatment of iris defects. The CUSTOMFLEX® ARTIFICIALIRIS is indicated for use in children and adults for the treatment of full or partial aniridia resulting from congenital aniridia, acquired defects, or other conditions associated with full or partial aniridia. When implanted, the CUSTOMFLEX® ...
-
Manufactured by Checkpoint Surgical Inc.based in USA
In surgery, every decision is critical, and every critical decision must be backed by information. CHECKPOINT GUARDIAN™ helps you unlock the information you need to assess and protect nerves during surgery?information that helps you make intraoperative decisions for your ...
-
Manufactured by Checkpoint Surgical Inc.based in USA
Checkpoint Surgical’s nerve stimulation devices inform intraoperative decision making to help surgeons safely and effectively protect, assess and repair motor nerves during surgeries. The CHECKPOINT Nerve Stimulator provides visual confirmation of nerve function and location in the surgical field, even in obscured anatomy. All Checkpoint stimulators may be used safely for sustained or ...
-
Manufactured by FareTec Inc.based in USA
The PST is field tested and outfitted with the most advanced materials available, this unit can serve a multitude of purposes. The PST quickly assembles into a stable patient aid platform for medics and doctors. Ideal for field hospitals and the like. The device is versatile and easy to transport for fast changing scenarios. AVAILABLE ON: GSA, DAPA & ...
-
Manufactured by Control Bionics, Inc.based in USA
The Uno Touch is a robust, lightweight speech generating device for people with communication disabilities. Designed to be easy to use in a variety of situations, the Uno Touch comes with an external wireless speaker and a shoulder strap. Easily control speaker volume, screen brightness, and other settings from the main screen. With the Uno Touch, gain access to a variety of software solutions ...
-
Manufactured by Mercury Biomedbased in USA
Safe induction of therapeutic hypothermia by anyone, anywhere, when it matters most. Inducing mild hypothermia in patients suffering sudden cardiac arrest, stroke and traumatic brain injury has been shown to reduce irreversible brain, vital tissue and organ damage – and the sooner, the ...
-
based in USA
Gastric acidity verification for NG/OG tube placement. The ONLY FDA cleared, CE Marked, CLIA waived product that uses pH to confirm gastric acidity for tubes intended to end in the stomach. RightBio Metrics pH indicators are a cost-effective way to confirm gastric acidity of tubes intended for the stomach. Published studies show pH can be used in place of x-ray to confirm initial placement. In ...
-
based in USA
Using pH to confirm NG/OG tube placement is Evidenced Based Best Practice. Immediate confirmation of gastric acidity for NG/OG tube placement, without exposure to x-ray. 2005 and 2010, 45% of all cases of harm caused by a misplaced NGT reported by the United Kingdom’s National Patient Safety Agency were due to misinterpreted X-rays. ...
-
Manufactured by Mercury Biomedbased in USA
Actively warming patients to achieve normal body temperature in the perioperative period is the standard of care. Hypothermia in the OR is associated with adverse outcomes including surgical site infections, impaired drug metabolism and cardiac ...
-
Manufactured by Sabre Industries Incbased in USA
Our Self-Supporting Structures are available with either tubular or solid round legs in three or four-leg models, they are capable of carrying moderate to heavy accessory loads. These towers feature construction step bolts on each leg to the 15′ spread level, and come individually packed with each set of section bracing clearly identified. In addition to offering tubular or solid round ...
-
Manufactured by Havalonbased in USA
REDI™ blades are made of high quality AUS8 steel and were engineered for everyday use. They can be resharpened and can stand up to all the tough tasks a traditional fixed blade can. What sets the REDI™ apart is, after extended use the blade can be replaced rather than needing to buy a whole new ...
-
Manufactured by Meridian Bioscience, Incbased in USA
Revogene is a flexible molecular platform capable of single analyte and multiplex testing that is packaged in a small footprint, and can help standardize testing throughout your health ...
-
Manufactured by Forge Biologics, Inc.based in USA
At Forge, our platform approach employs the triple plasmid transfection method – the industry-standard method for manufacturing AAV. This method is bolstered by Forge’s proprietary technologies, our Ignition Cells™ and pEMBR™ adenovirus helper plasmid. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you