Venous Pressure Equipment & Supplies
-
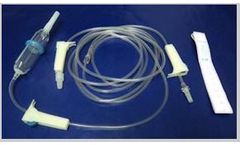
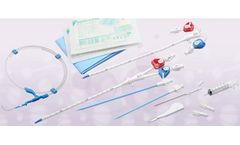
Manufactured by Laboratorios Yermedic SACbased in PERU
Top set length of 150 cm. Measuring length of tubing 95 to 125 cm. Length reading tape measure central venous pressure semirigida and graduated to 35 + / - 5. Sterile Packaging: medical grade paper envelope with transparent ...
-
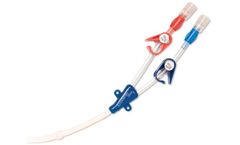
Manufactured by Mirador Biomedicalbased in USA
The Compass Vascular Access Lock facilitates Central Venous Pressure (CVP) monitoring and is ideally suited for use in the Emergency department to manage the septic patient in accordance with the Early Goal Directed Therapy protocol. The Compass VA Lock can be attached directly to the central venous catheter, providing a central ...
-
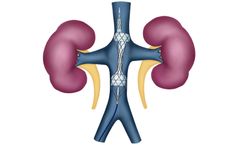
Manufactured by Magenta Medical Ltd.based in ISRAEL
As encapsulated organs, they are particularly vulnerable to congestion: cardiac dysfunction and fluid buildup/redistribution leads to increased central venous pressure, increased renal venous pressures and, ultimately, increased renal interstitial pressures. The latter result in decreased renal blood flow, ...
-
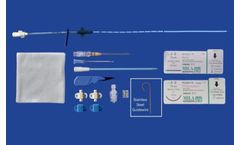
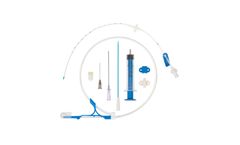
Manufactured by MILA International, Inc.based in USA
Indicated to provide central venous access for delivery of fluids and medications and blood sampling. Other uses include measuring central venous pressure and administration of hypertonic solutions (such as TPN). Contraindicated in coagulopathic patients. Always use a dedicated lumen for blood sampling if ...
-
Manufactured by DAESUNG MAREF CO., LTD.based in SOUTH KOREA
DVT-PRO the intermittent pneumatic compression system device helps to prevent the occurrence of DVT (Deep Venous Thrombosis) by pressurizing legs using air pressure and so improving the blood circulation of a user. A device intended to prevent CVT/PE by increasing venous blood flow to a patient who has a risk factor of DVT/PE. ...
-
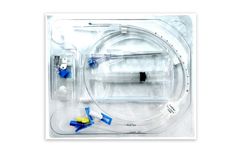
Manufactured by Cook Medical LLCbased in USA
The central venous catheter is designed for treatment of critically ill patients and is suggested for: 1. Continuous or intermittent drug infusions; 2. Central venous blood pressure monitoring (CVP); 3. Acute hyperalimentation; 4. Blood sampling; 5. ...
-
Manufactured by Ergotrics NVbased in BELGIUM
This reduces the risk of bedsores, reduces Intra-Abdominal Pressure (IAP) and prevents venous congestion. The use of compressed air instead of manual labour is an ergonomic breakthrough in the ...
-
Manufactured by Unilenebased in USA
The variety of lumens lumens give us dedicated to infusion therapy, the monitoring of pressure, and venous sampling. The CVC is next to a set of components required for percutaneous insertion. The whole set is sterilized by exposure to ethylene ...
-
Manufactured by Bioteque Corporationbased in TAIWAN
Thermo-sensitive wings, rotatable wings allows easy turning for right direction after ...
-
Manufactured by AngioDynamics, Inc.based in USA
Providing Even More Durability and Site Care Options. Luminal Design Allows for High Flow. Count on the 14.5 French EVENMORE catheter from AngioDynamics to deliver even greater performance and the care your patient deserves. Engineered for durability, it combines all the attributes that are basic to the very best dialysis treatment and patient ...
-
Manufactured by Avery Dennison Medicalbased in USA
Polyurethane Foam/Film adhesive border dressings are hydrophilic, medical grade polyurethane (PU) foams made of a soft textured material, designed to handle high volumes of wound exudate and provide a comfortable wear for the patient. The product has a high moisture vapor permeability and is available with a moisture- and bacteria-resistant outer PU film layer. ...
-
Manufactured by Milliken & Companybased in USA
AGILE dressings were developed to meet the unmet needs of patients and clinicians for a highly absorbent gellable dressing with increased structural integrity and conformability. AGILE dressings combine the patented Active Fluid Management® technology with an innovative weave of gelling fibrils. AGILE has the unique ability to pull excess moisture away from the wound bed, absorbs it into the ...
-
Manufactured by Avery Dennison Medicalbased in USA
The five-layer foam dressing is an absorbent, self-adherent, soft silicone wound dressing available in a border and non-border version. It comprises of a breathable silicone wound contact layer designed for atraumatic removal, a polyurethane foam and superabsorbent fiber composite pad with high absorption capacity and a vapor-permeable, water- and bacteria-resistant polyurethane film outer layer. ...
-
Manufactured by ELCAT GmbHbased in GERMANY
Dual channel Photoplethysmograph for venous and arterial, functional diagnosis, intuitive handling (10,1“ Touch Display), quick and highly diagnostic, quantitative Photoplethysmographie, automatic calibration, PAD screening via ABI quick test, Optimised sensors for venous and arterial diagnosis, Integrated evaluation software for easier result assessment, ...
-
Manufactured by Prodimedbased in FRANCE
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
-
Manufactured by ACI Medical, LLCbased in USA
The APG® Air Plethysmograph is a non-invasive diagnostic tool that quantifies the physiological components of chronic venous disease including: Chronic obstruction; Valvular reflux; Calf muscle pump function; Venous hypertension. The APG® Air Plethysmograph is the only system that measures true blood volume changes in milliliters and blood flow in ...
-
Manufactured by CSL Behringbased in USA
It is a sterile, preservative-free 20% w/v human albumin solution. It has a nominal osmolality of 130 mOsra/kg, is hypotonic and the pH is 6.7 to 7.3. It is hyperoncotic and hypo-osmotic compared to human serum. Albumex 20 is manufactured from human plasma collected by Australian Red Cross Lifeblood. It is prepared using predominantly chromatographic ...
-
Manufactured by Speciality Fibres and Materials Ltd. (SFM)based in UNITED KINGDOM
Effective against bacteria such as E. Coli, Staphylococcus Aureus and Yeast (Candida Albicans). Our Silver Calcium Alginates fabric can be supplied in roll form, slit reels or cut pieces, packaged and sterile. various GSM available. High absorbency, High conformability, Antibacterial performance*, Strong dressing, Reduced fibre shed, various GSM and cut sizes available on request, Biodegradable ...
-
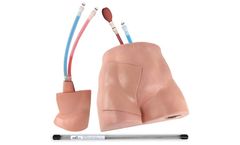
Manufactured by Simulab Corporationbased in USA
Simulab's FemoraLineMan System is an ultrasound-compatible femoral line vascular access trainer. The product offers an effective training solution for central venous or arterial access at the femoral site. ...
-
Manufactured by Simulab Corporationbased in USA
Simulab's FemoraLineMan Training Package is an ultrasound-compatible task trainer that offers an effective training solution for central venous or arterial access using the femoral site. This femoral line trainer uses the same patented technology as the highly acclaimed TraumaMan System and allows medical professionals to train using real-time ultrasound guidance during ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you