Preclinical Trial Equipment & Supplies
-
by PSC Biotech®based in NORTH AMERICA
As a leading biotechnology company, MtoZ Biolabs is committed to providing comprehensive, one-stop CRO testing services to scientific research institutions and pharmaceutical companies worldwide. Whether in the early stages of drug discovery, candidate drug evaluation, or preclinical development, MtoZ Biolabs can provide professional technical support and consulting services to ...
-
Manufactured by CanaQuest Medical Corp.based in CANADA
Based on positive results from the preclinical research, we are ready to move forward with a clinical trial to see how well it works in humans. Clinical trials happen in several phases during which different questions are asked. Each phase builds on the results of previous ...
-
Manufactured by Kuros Biosciences A.G.based in SWITZERLAND
MagnetOs isn’t like other bone grafts. It grows bone even in soft tissue thanks to our unique NeedleGrip™ surface technology which provides traction for our body’s vitally important ‘pro-healing’ immune cells (M2 macrophages).*†6,7 This in turn, unlocks previously untapped potential to stimulate stem cells – and form new bone throughout the graft.*8-10 ...
-
Manufactured by Precision X-Raybased in USA
Catering to molecular biologists and imaging physicists alike, these systems represent the pinnacle of versatility and precision in preclinical radiation therapy ...
-
Manufactured by Precision NanoSystemsbased in CANADA
The system can manufacture between 10 mL and 10 L of formulation which allows researchers to use the Blaze for preclinical toxicology testing, and for early Chemistry, Manufacturing and Controls (CMC) ...
-
Manufactured by Aptevo Therapeuticsbased in USA
ADAPTIR-FLEX technology extends advantages of ADAPTIR technology to create protein therapeutics with varied specificity and valency and potentially new modes of ...
-
by OSSIO Incbased in USA
OSSIOfiber® Cannulated and Solid-Core Fixation Nails allow surgeons to offer patients a strong and biointegrative solution for a wide range of surgical techniques. Comprised of OSSIOfiber® Intelligent Bone Regeneration Technology, Fully integrates into native anatomy without adverse inflammation,, as proven in pre-clinical studies, Rivals performance of metal headless cannulated ...
-
Manufactured by Ito Co., Ltd.based in JAPAN
High-end Combination Unit with 5 Independent Channels and Body Diagram ...
-
Manufactured by Kiadis Pharma NVbased in NETHERLANDS
Alomfilimab SAR445256 (formerly KY1044) is a human monoclonal IgG1 that selectively binds to Inducible T cell CO-stimulator (ICOS), a protein expressed at high levels on immunosuppressive regulatory T cells and at lower levels on effector T cells. Alomfilimab is designed to exert anti-tumour activity through preferential depletion of intra-tumoral regulatory T cells and stimulation (agonism) of ...
-
Manufactured by Sequana Medical NVbased in BELGIUM
Direct Sodium Removal (DSR®) therapy is Sequana Medical’s novel and proprietary approach under development for the management of fluid overload due to heart failure. The sodium concentration in patients with fluid overload is in balance but there is too much sodium and too much fluid in the body. Sequana Medical’s approach is to remove excess sodium in patients with ...
-
Manufactured by eTheRNAbased in BELGIUM
Our mRNA platforms and vast in-house experience help partners quickly optimize, screen and select an mRNA code, develop a candidate, and ensure effective & prime manufacturing of the mRNA from research grade to GMP scale. Our platform’s scale and flexibility is uniquely suited to respond to the needs of development partners across a wide range of therapeutic ...
-
Manufactured by CN Biobased in UNITED KINGDOM
Our Non-Alcoholic Fatty Liver Disorder (NAFLD)/NASH in vitro Services offer a preclinical tool to test therapeutics in a human-relevant model, replicating key disease phenotypes including fibrosis and reporting clinical biomarkers to allow translatability to clinical trials. ...
-
Manufactured by PhenoSys GmbHbased in GERMANY
The optomotor response (OMR) is a reflex used to assess visual function. The PhenoSys qOMR (quantitative optomotor response) is a unique system that automatically measures the OMR with minimal experimenter effort. It uses a virtual stimulation cylinder that continuously aligns with the animal´s head position. Based on real-time head tracking, quantitative OMR measurements run fully ...
-
Manufactured by Aptevo Therapeuticsbased in USA
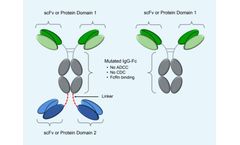
Demonstrated ability to produce monospecific and bispecific drug candidates; Leverages IgG1 Fc to form homodimeric bispecific molecules; IgG1-derived Fc can be mutated to ablate effector functions antibody-dependent cellcytotoxicity (ADCC) and complementdependent cytotoxicity (CDC), while maintaining original stability and half-life (confirmed by cell binding, cell function, SPRbased measurements ...
-
Manufactured by Gyros Protein Technologies ABbased in SWEDEN
Gyrolab® xPand immunoassay system is packed with essential functions requested by users wanting greater flexibility, higher throughput, unattended operation and rapid analysis of large sample numbers. Analyze up to five CDs unattended – generate up to 560 data points within five hours. Cooling of samples and reagents. Mix and match methods/CDs in a single run. Flexible run setup & ...
-
Manufactured by Mersana Therapeuticsbased in USA
Once ADCs release their cytotoxic payload into targeted cells, the drug is often able to cross cell membranes, entering and potentially killing neighboring cells whether those cells are cancerous or not. This effect is called bystander-killing, which is advantageous when bystander cells are cancerous but toxic if the cytotoxic drug is able to enter adjacent healthy cells. The DolaLock payload ...
-
Manufactured by MorphoSys AGbased in GERMANY
Constellation Pharmaceuticals, a MorphoSys company, is using a starting dose of 125 mg in MANIFEST, our global, multicenter, open-label Phase 2 study of pelabresib in patients with Myelofibrosis (MF). Preclinical studies and translational insights from our first-in-human study of pelabresib led us to prioritize the clinical development of pelabresib in ...
-
Manufactured by Camurus ABbased in SWEDEN
FluidCrysta nanoparticles can resolve bioavailability issues for water and lipid-soluble pharmaceuticals or biodegradation-sensitive drugs, such as peptides and proteins. FluidCrystal nanoparticles are usually water-based and comprise a stable emulsion of nanoparticles with a liquid crystalline structure. Investigational medicinal products based on this technology are administered either ...
-
Manufactured by AVITA Medicalbased in USA
Epidermolysis Bullosa (EB) is a group of rare and incurable skin disorders caused by mutations in genes encoding structural proteins resulting in skin fragility and blistering, leading to chronic wounds and, in some sub-types, an increased risk of squamous cell carcinoma or death. Dystrophic EB is estimated to affect 3-8 million people[1], and there is currently no FDA-approved ...
-
Distributed by American Laboratory Products Company (ALPCO)based in INDIA
We offer a range of research tools required to identify novel Cardiovascular Disease and Oxidative Health biomarkers, enabling scientists to test their relevance in basic, preclinical and clinical studies. Cardiovascular Disease (CVD) encompasses a constellation of diseases related to the heart and/or circulatory system. Two primary risk factors for CVD include obesity and Type 2 ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you