Preclinical Trial Equipment & Supplies
-
Manufactured by Auris Medical AGbased in SWITZERLAND
AM-125 for the treatment of vertigo is expected to complete its Phase 2 TRAVERS trial in Q2 2022 and to move into Phase 3 in Q4 2022. Keyzilen® (AM-101) for the treatment of acute inner ear tinnitus and Sonsuvi® (AM-111) for the treatment of acute inner ear hearing loss have both been evaluated in Phase 3 trials, but will require further clinical testing. ...
-
Manufactured by PlasmidFactory GmbH & Co. KGbased in GERMANY
Plasmid DNA in reproducible and certified quality for preclinical and veterinary research. Cultivation without use of animal derived media. Controlled removal of bacterial LPS endotoxins. Complete removal of RNA and proteins. A large number of quality tests already inclusive – without any additional ...
-
Manufactured by Mirus Bio LLCbased in USA
TransIT-VirusGEN SELECT Transfection Reagent is designed to enhance delivery of packaging and transfer vector DNA to suspension and adherent HEK 293 cell types in order to increase production of recombinant lentivirus and adeno-associated virus (AAV). Key benefits of TransIT-VirusGEN SELECT Transfection Reagent include: Performance – Efficient DNA delivery for large-scale production of ...
-
Manufactured by AOBiome Therapeutic, LLCbased in USA
We have completed six Phase 2 clinical programs and multiple pre-clinical programs. Over 1,000 patients have been enrolled in our clinical trials since 2016. All of those patients except for 28 in our open label pediatric study have been enrolled in randomized double-blind placebo controlled clinical trials evaluating both topical and intranasal routes of ...
-
based in USA
Radiation exposure of researchers is blocked with internal protection, making the InAlyzer safe, effective, and accurate for use in the preclinical research phase. Save time, cost, and animals, Automated, accurate, and precise measurement, High resolution images ...
-
based in USA
Our proprietary GalXC technology leverages a naturally occurring biologic process, ribonucleic acid interference, RNAi, to create therapies that silence disease-causing genes. This platform enables Dicerna to create molecules that can effectively interfere with the RNAi processes that lead to defective or misregulated proteins that cause disease. ...
-
Manufactured by eTheRNAbased in BELGIUM
Therapeutic area: cancer vaccines; Systemic, intravenous (IV) ...
-
Manufactured by Enteromebased in FRANCE
New generation of oral bioactives to treat inflammatory and autoimmune diseases. EndoMimicsTM are first-in-class orally available bioactives drugs based on proteins secreted by the gut bacteria that act like human hormones or cytokines. Enterome expects partnering to play a central in generating value from its EndoMimics pipeline. ...
-
by XELTIS BVbased in NETHERLANDS
Fully synthetic restorative pulmonary heart valve for Right Ventricular Outflow Tract reconstruction, overtime turning into a living heart valve made of patient’s own ...
-
Manufactured by Auro Vaccines LLCbased in USA
Auro Vaccines has developed a VesiculoVax™-vectored vaccine to protect against the arthralgic disease caused by infection with Chikungunya ...
-
by Verb Bioticsbased in USA
Yso3 is a preclinical drug development program aimed at treating asthma. Using a revolutionary form of living biotherapy targeting the lung, YSOPIA is pioneering the innovative field of the lung microbiome. As part of this program, YSOPIA has selected a clinical candidate having demonstrated strong in vivo efficacy on inflammation and lung functions. ...
-
Manufactured by Citagenix Inc.based in CANADA
Neomem® FlexPlus is a single layer collagen membrane derived from porcine peritoneum. Suture pullout testing has shown this membrane to be biomechanically strong yet conformability evaluations demonstrate the ability to adapt to the contour of the surgical site. Neomem® FlexPlus hydrates quickly and handling characteristics make surgical placement easy. It can be repositioned easily and ...
-
based in USA
Selecta’s first ImmTORTM + gene therapy candidate MMA-101 for methylmalonic acidemia (MMA) is expected to enter clinical trials the end of 2021. MMA is a rare metabolic disease that may lead to metabolic acidosis and hyperammonemia and is associated with long-term complications including feeding problems, developmental delay, intellectual disability, and chronic kidney ...
-
Manufactured by Kiadis Pharma NVbased in NETHERLANDS
K-NK00X: Kiadis is evaluating preclinical programs with K-NK-cell therapies for the treatment of hematologic and solid ...
-
Manufactured by Capricor Therapeutics, Inc.based in USA
Since the initial publication in 2007, CDCs have been the subject of over 100 peer-reviewed scientific publications and have been administered to approximately 200 human subjects across several clinical trials. CAP-1002 consists of allogeneic “off-the-shelf” cardiosphere-derived cells, or CDCs, a type of cardiac cell therapy that has been shown in pre-clinical and ...
-
Manufactured by Jointechlabs Inc.based in USA
Based on our platform and technology, Jointechlabs is developing therapies for unmet medical needs. Our first therapeutic in clinical development JTL-T-01 is a proprietary injectable stem cell scaffold as a biologic therapy for osteoarthritis, for approval under the FDA’s fast-track program. JTL-T-01 has been designed as an autologous point-of-care therapy enabling the patient’s own ...
-
Manufactured by Brenus Pharmabased in FRANCE
An allogeneic first in class immunotherapy harnessing the patient immune system to fight immune tolerance and prevent cancer ...
-
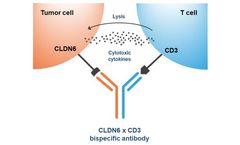
Manufactured by Context Therapeutics Inc.based in USA
There is growing interest in applying antibody modalities including bispecifics, antibody-drug conjugates, and CART to solid tumors. However, identifying appropriate tumor-specific targets that avoid adverse effects in healthy tissue has been challenging. The tight junction protein Claudin 6 (CLDN6) is a validated therapeutic target for many solid tumor types, including ovarian, endometrial, ...
-
Manufactured by Eclipse Regenesisbased in USA
The Eclipse XL1 is an entirely mechanical and repeatable approach designed to grow healthy intestinal tissue 2-3x longer within 2-3 weeks. Early studies have shown that new tissue has begun to function and absorb nutrients equally (or greater) than existing healthy intestinal ...
-
based in USA
Advancement of extracellular vesicle therapy to the patient has been hampered by an inability to isolate extracellular vesicles in useful quantities while preserving their structural and functional integrity. EVs derived from MSCs enjoy the same immunosuppressant characteristics of their parent cells. However, when EVs are processed incorrectly, they can trigger an adverse inflammation response ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you