Preclinical Trial Equipment & Supplies
-
Manufactured by Neuropathix, Inc.based in USA
Neuropathix has focused its science on the research and development of a pipeline of next generation socially responsible pain management ...
-
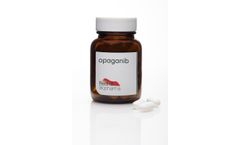
Manufactured by RedHill Biopharma Ltd.based in ISRAEL
Opaganib (ABC294640) is a first-in-class, proprietary sphingosine kinase-2 (SK2) selective inhibitor, administered orally, with anticancer, anti-inflammatory and anti-viral ...
-
Manufactured by AIVITA Biomedical, Inc.based in USA
AIVITA Biomedical is using its patient-specific vaccine platform to develop a rapidly scalable vaccine candidate, AV-COVID-19, for the novel coronavirus (SARS-CoV-2). AIVITA’s personalized vaccine begins with a basic blood draw, from which the recipient’s own immune cells are extracted and loaded with SARS-CoV-2 antigen. The resulting personalized treatment is designed to create ...
-
by Endomimeticsbased in USA
In many medical settings, devices are commonly used to help doctors treat patients. For example, stents are often used to open blocked arteries, repair torn vessels, and even to keep nasal passages from swelling shut after surgery. Unfortunately these devices fail often resulting in additional surgeries and often replacements of the original ...
-
Manufactured by Vascudyne, Inc.based in USA
Synthetic vascular grafts currently used in clinic have poor long term outcomes and low patency rates. Hyperplasia, calcification, foreign body immune response, infection, and atheroma formation are commonly encountered limiting the use of synthetic grafts, with none available for small diameter applications like coronary ...
-
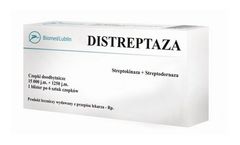
Manufactured by Biomed Lublin S.A.based in POLAND
Name Of The Medicinal Product; DISTREPTAZA; Streptokinase + Streptodornase; 15000 IU + 1250 IU. Rectal ...
-
Manufactured by emka Technologies S.A.Sbased in FRANCE
easyTEL+ is a fully implantable digital system, that transmits physiological data from conscious freely moving laboratory animals. It can be remotely managed and configured. ...
-
Manufactured by Lattice Medicalbased in FRANCE
Lattice Medical is a biomedical start-up that was launched in October 2017; Patented 3D technology developed with CHU Lille-France that allows adipose tissue to naturally ...
-
Manufactured by Biotron Limitedbased in AUSTRALIA
Biotron is developing novel small molecule antiviral therapeutics targeting a range of viruses including Hepatitis C virus (HCV) and ...
-
Manufactured by Scopus BioPharmabased in USA
Our licensed gene therapy, CO-sTiRNA™, is a dual-action STAT3 inhibitor. STAT3 is a gene that drives tumor cell growth and anti-tumor immune suppression. CO-sTiRNA™ is a highly selective and targeted gene therapy that silences the activity of the STAT3 gene by way of RNA interference. CO-sTiRNA™ also stimulates TLR9 receptors to activate the body’s immune defense to ...
-
Manufactured by SafeHealbased in FRANCE
Minimally invasive and fully reversible, Colovac is anchored by a vacuum seal above the anastomosis and can be removed during a routine endoscopic procedure. Clinical trials support Colovac’s effectiveness in improving and expediting patients’ recoveries from surgery. This is an especially important benefit for surgeons, clinicians, payors and postoperative care ...
-
Manufactured by CervoMedbased in USA
Our lead product candidate, Trans sodium crocetinate (TSC), is being developed to enhance the diffusion of oxygen to tissues with low oxygen levels, also known as hypoxia, a serious complication of many of medicine’s most intractable and difficult-to-treat conditions. ...
-
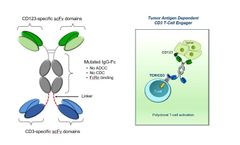
Manufactured by Aptevo Therapeuticsbased in USA
APVO436 is an optimized ADAPTIR bispecific antibody candidate designed to redirect T-cell cytotoxicity through the dual targeting of CD3, a T-cell co-receptor that promotes cytotoxicity, and CD123, a cell surface receptor highly expressed in several hematological malignancies, including acute myeloid leukemia, acute lymphoblastic leukemia, hairy cell leukemia, myelodysplastic syndrome, and ...
-
based in POLAND
Neuromyelitis optica (NMO) is an neurological autoimmune condition classified as a rare disease. Because of this classification, NMO lacks effective treatment options, since it is not of primary interest to the pharmaceutical industry. However, considerable social and institutional support exists for developing new therapies for such rare diseases. The PB002 (AptaPheresis) project aims to ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you