Oncology Equipment & Supplies
-
Manufactured by Panacea Medical Technologies Pvt. Ltd.based in INDIA
Effective simulation and verification are of primary importance before treatment delivery to ensure safe deliver of radiation. At Panacea, we believe working together to break down boundaries and deliver seamless approach to cancer care. IMAGIN, an advanced flat panel radiotherapy simulator delivers the most efficacious simulation by optimized target delineation, target margins and target ...
-
based in USA
APH-0912 is a combination of a Taxotere® prodrug and monoclonal antibody targeted to treat metastatic cancer. The prodrug is a combination of a docetaxel molecule, the potent anticancer drug, and a sugar molecule to enhance solubility covalently linked to a tumor-specific antibody. This molecular combination is not bioactive prior to administration. After infusion and adsorption onto targeted ...
-
Manufactured by BridgeBio Pharma, Inc.based in USA
BridgeBio is developing SHP2 inhibitors as potentially effective additions to the therapeutic arsenal for difficult-to-treat cancers. SHP2, encoded by the PTPN11 gene, links growth factor signaling with the downstream RAS/ERK/MAPK pathway to regulate cell growth and division. Over-activity of this pathway, often driven by distinct gene mutations, causes or contributes to many human cancers. ...
-
based in USA
Despite the widespread use of the 5-HT3 receptor antagonist anti-emetics such as Palonosetron (Aloxi; Eisai), Ondansetron (Zofran®; GSK), Granisetron (Kytril®; Roche Pharmaceuticals), and Dolasetron (Anzemet®; Aventis), CINV continues to be reported by up to 70% of adult patients receiving highly emetogenic chemotherapy agents and 58% of school-age and adolescent-age children ...
-
Manufactured by ISO Medbased in FRANCE
FB Medical offers a complete range of Huber Needles with extensions set and movable wings called Onco-Grip. Onco-Grip are individualy packaged in a rigid blister pack and sterilized with ethylene ...
-
Manufactured by Daiichi Sankyo Company Limitedbased in JAPAN
TURALIO (pexidartinib) is an oral small molecule that targets colony stimulating factor 1 receptor (CSF1R), KIT proto-oncogene receptor tyrosine kinase (KIT), and FMS-like tyrosine kinase 3 (FLT3) harboring an internal tandem duplication (ITD) ...
-
Manufactured by ISO Medbased in FRANCE
ISO Med Huber Needles are available with a luer lock hub. Their unique point design promotes easy penetration and maximum life for the port septum.They are non-siliconized to minimise "back out". ISO Med offers, under the FB Medical brand, a complete range of Huber Needles, straight or 90° curved. They are designed for single use and easy insertion into vascular access device, and may be used ...
-
Manufactured by ISO Medbased in FRANCE
FB Medical offers a complete range of Huber Needles with extensions sets. Extension sets are individualy packaged in a rigid blister pack and sterilized with ethylene ...
-
Manufactured by Panacea Medical Technologies Pvt. Ltd.based in INDIA
Impact Sigma Board is a unique setup for patient positioning which is well designed for complete body positioning with accuracy. It is one board for all your needs. Light weight and Carbonfiber made, Easy Patient setup, One solution for whole body positioning ...
-
Manufactured by Daiichi Sankyo Company Limitedbased in JAPAN
ENHERTU (5.4 mg/kg) is approved in more than 50 countries for the treatment of adult patients with unresectable or metastatic HER2 positive breast cancer who have received a (or one or more) prior antiHER2-based regimen, either in the metastatic setting or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within six months of completing therapy based on the ...
-
Manufactured by ISO Medbased in FRANCE
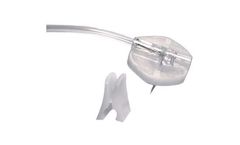
The INFU-KT is a complete implantable access system for patients who require repeated delivery of cancer chemotherapy agents, parenteral nutrition, antibiotics, blood products and for blood sampling. The injections are less painful and the implanted port allows the patient to lead a normal day life between infusions. The INFU-KT port consists of a compact titanium injection port, with a ...
-
Manufactured by Biocartis NVbased in BELGIUM
The fully automated Idylla KRAS Mutation Test covers 21 KRAS mutations in exons 2,3 and 4 showing an excellent concordance to reference methods of >95%. Comparison with various other KRAS detecting technologies revealed a superior performance of the IdyllaTM KRAS Mutation Test in terms of ease of use, turnaround time, and hands-on time while demonstrating superior levels of ...
-
Manufactured by ISO Medbased in FRANCE
This is a range of implantable titanium ports available either individually or in kit form. The INFUKT-CT ports are designed to allow the power injection of contrast during imaging ...
-
Manufactured by Daiichi Sankyo Company Limitedbased in JAPAN
VANFLYTA is a FLT3 inhibitor approved in Japan for the treatment of adult patients with acute myeloid leukemia (AML) that is FLT3-ITD mutation positive, including for use in combination with standard cytarabine and anthracycline induction and standard cytarabine consolidation chemotherapy and as maintenance monotherapy for newly diagnosed FLT3-ITD positive AML, based on the results of the ...
-
Manufactured by Biocartis NVbased in BELGIUM
The fully automated Idylla ctKRAS Mutation Test is minimally invasive, fast and easy to perform and can be used as complement to tissue testing to determine the RAS mutation status at diagnosis. IdyllaTM ctKRAS covers 21 KRAS mutations in exons 2,3 and 4 and showed an high concordance to NGS of 96% using plasma samples from 198 mCRC patients enrolled in the prospective multicenter RASANC ...
-
Manufactured by Daiichi Sankyo Company Limitedbased in JAPAN
INJECTAFER (ferric carboxymaltose injection) is indicated for the treatment of iron deficiency in adult patients with heart failure; iron deficiency anemia (IDA) in adult and pediatric patients 1 year of age and older who have either intolerance to oral iron or an unsatisfactory response to oral iron; and adult patients with IDA who have non-dialysis dependent chronic kidney ...
-
Manufactured by ISO Medbased in FRANCE
FB Medical offers a complete range of Huber Needles with extensions set and movable wings called Onco-Grip Safe. Onco-Grip Safe are individualy packaged in a rigid blister pack and sterilized with ethylene ...
-
Manufactured by Biocartis NVbased in BELGIUM
The fully automated Idylla EGFR Mutation Test covers 51 mutations in exons 18–21 in a single cartridge using only 1 FFPE tissue section from metastatic NSCLC showing a high concordance of >95% compared with reference ...
-
Manufactured by Matek Medikalbased in TURKEY
Matek Bone Biopsy Needle allows easy and safe entry with its ergonomically designed handle, sharp needle point and adjustable needle ...
-
Manufactured by Matek Medikalbased in TURKEY
Captura Bone Marrow Biopsy Set allows easy, safe and fast procedure to the marrow cavity with its ergonomically designed handle, sharp needle point and Capture ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you