Oncology Equipment Supplied In Europe
-
Manufactured by Olink Proteomicsbased in SWEDEN
The most comprehensive, high-performance protein biomarker panels available for oncology studies. The flexibility and scalability of our Olink® Explore platform can be utilized either to screen our entire library of 3072 assays, or in studies that focus on one or several of the eight Explore 384-plex panels that make up the Explore 3072 protein library. ...
-
Manufactured by AJ Vaccines A/Sbased in DENMARK
VesiCulture (BCG Culture) For the treatment of Bladder ...
-
Distributed by GPC Pharma Ecza Deposu San. Tic. Ltd. Sti.based in TURKEY
In addition to the traditional drugs used in the treatment of oncological diseases, the frequency of which is increasing day by day, we supply global oncological drugs with smart drug technology and deliver them to the patients under favorable ...
-
Manufactured by Hemodia SASbased in FRANCE
From the patient's preparation to the care: Syringes, needle packs; Specific implanted ports care kits dedicated to needle installation, infusion connection and disconnection; Chemotherapy decontamination kits: for carers handling cytotoxic agents. Our packs regroup all devices and consumables required for preparing and carrying out an intravenous chemotherapy session. To be totally in line with ...
-
Manufactured by Air Sentry Limitedbased in UNITED KINGDOM
The Air Sentry HEPA Air Purifier system is a flexible, modular system. It offers Positive Air Pressure solutions for Oncology & Radiology Depts which care for the most immunosuppressed ...
-
Manufactured by AstraZenecabased in UNITED KINGDOM
Mechanism: ATM inhibitor; Area under investigation: glioblastoma; Date commenced phase: Q2 2018; Estimated Filing Acceptance: US: EU: Japan: China. Additional information. Molecule size: Small molecule Status ...
-
Manufactured by The Menarini Groupbased in ITALY
TAGRAXOFUSP is a novel targeted therapy directed to the IL-3 receptor α (CD123), a target present on a wide range of malignancies including Blastic Plasmacytoid Dendritic Nell Neoplasm (BPDCN). It comprises human IL-3 recombinantly fused to a truncated diphtheria toxin (DT) payload engineered such that IL-3 replaces the native DT receptor-binding domain. The IL-3 domain of TAGRAXOFUSP ...
-
Manufactured by Teknimedbased in FRANCE
F20® radiopaque bone cement is a ready to use, medium viscosity, low exothermic, self-curing bone cement with a high content of radiopaque agent for a perfect level of visualization and safety required during percutaneous vertebral bone augmentation procedures. Its composition has been optimized to get the most suitable handling properties for surgeon’s convenience. F20® comes as an ...
-
Manufactured by AstraZenecabased in UNITED KINGDOM
Mechanism: CDK9 inhibitor Area under investigation: haematalogical malignancies Date commenced phase: Q4 2017 Estimated Filing Acceptance: US: EU: Japan: China. Additional information. Molecule size: Small molecule Status change: New to Phase ...
-
Manufactured by The Menarini Groupbased in ITALY
MEN1703 is a dual kinase inhibitor targeting PIM (PIM1, PIM2, PIM 3) and FLT3 kinases. FLT3 internal tandem duplication (FLT3-ITD) is one of the most common genetic lesions in acute myeloid leukemia patients (AML) and is associated with a poor prognosis. PIM kinases are thought to be one of the major drivers of the resistance phenotype to FLT3 inhibitors and their inhibition in relapsed samples ...
-
Manufactured by The Menarini Groupbased in ITALY
FELEZONEXOR (SL-801) is a novel, oral, small-molecule reversible inhibitor of exportin-1 (XPO1), a key nuclear transport protein. XPO1 is overexpressed by many cancers, leading to mislocalization of key tumor suppressors and growth-regulatory proteins in the cytoplasm. FELEZONEXOR is currently being tested as a potential treatment for patients with solid ...
-
Manufactured by AstraZenecabased in UNITED KINGDOM
Mechanism: BCL2/xL; Area under investigation: haematological malignancies; Date commenced phase: Q4 2019; Estimated Filing Acceptance: US: EU: Japan: China; Additional information: Partnered product. Molecule size: Small molecule; Status ...
-
Manufactured by The Menarini Groupbased in ITALY
NEXPOVIO is a first-in-class, oral exportin 1 (XPO1) inhibitor. XPO1 is a nuclear exporter protein that transport from the nucleus to the cytoplasm several proteins, including tumor suppressors, oncogenes, and proteins involved in governing cell growth; it is often overexpressed, and its function mis-regulated in several types of cancers. By inhibiting the XPO1 protein, tumor suppressors proteins ...
-
Manufactured by The Menarini Groupbased in ITALY
MEN1309 is an antibody-drug conjugate (ADC) constituted by a fully human IgG1 antibody conjugated through a cleavable linker to a cytotoxic agent (a maytansin derivative, DM4), responsible for its antitumor activity. The target antigen, CD205, is highly expressed in a wide range of both solid and hematological cancer cells. Once MEN1309 binds its target, the MEN1309/CD205 complex is rapidly ...
-
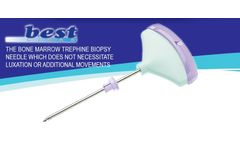
Manufactured by STERYLAB S.r.l.based in ITALY
The design of BEST-LISAS® is the most advanced to date and guarantees to the physician the following ...
-
based in ITALY
AKYNZEO® (netupitant/palonosetron) is the first and only fixed-dose antiemetic in combining the new NK-1 antagonist netupitant with the sole antagonist of the second generation of serotonin, palonosetron, already marketed by Italfarmaco since 2006 as ALOXI®. Thanks to its totally innovative oral formulation and to the characteristics of its active ingredients, which act in a synergistic ...
-
based in ITALY
MYELOSTIM® (lenograstim) is the only glycosylated granulocyte growth factor, indistinguishable from the native human one, effectively enabling to prevent and control bone marrow toxicity caused by the use of chemotherapeutic agents producing neutropenia, and thus facilitating its administration according to the time and doses as scheduled by the ...
-
Manufactured by Aries Srlbased in ITALY
Protective bags for vial, bags and syringes containing light sensitive solutions. Materials: polyethylene LD. Latex free – pvc free. Produced with a special barrier effect for light source with wave length between 200 and 600 nm. The optic filter allows to block selectively the wave length responsible for the inactivation of light sensitive solutions. The transparency of the material allows ...
-
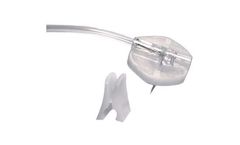
Manufactured by ISO Medbased in FRANCE
FB Medical offers a complete range of Huber Needles with extensions set and movable wings called Onco-Grip. Onco-Grip are individualy packaged in a rigid blister pack and sterilized with ethylene ...
-
Manufactured by ISO Medbased in FRANCE
ISO Med Huber Needles are available with a luer lock hub. Their unique point design promotes easy penetration and maximum life for the port septum.They are non-siliconized to minimise "back out". ISO Med offers, under the FB Medical brand, a complete range of Huber Needles, straight or 90° curved. They are designed for single use and easy insertion into vascular access device, and may be used ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you