Medical Sterilization System Equipment & Supplies
-
Manufactured by Nordion (Canada) Inc.based in CANADA
The Parallel Row Pallet Irradiator provides the ideal solution for processing intact pallets of product, reducing material handling labour cost while maximizing throughput. Ideal for both phytosanitary applications and medical device sterilization, the Pallet Irradiator accommodates a variety of product densities and dose uniformity requirements. The Pallet ...
-
Manufactured by Wuhan Dico Chemical Co., Ltd.based in CHINA
The company contains 2.00-2.50% glutaraldehyde and synergist sodium nitrite and sodium bicarbonate. It can kill pathogenic entero becteria, pyogenic coccus and bacterial ...
-
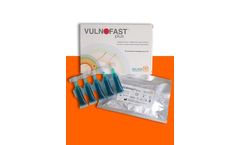
Manufactured by Molteni Therapeutics s.r.lbased in ITALY
VULNOFAST® plus is an improved formulation of VULNOFAST® gel 0.3%. VULNOFAST® plus is a sterile medical device (CE0373) topically administered recommended as an adjuvant for the local treatment of skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a red light source VULNOLIGHT® (led source at 630 ...
-
Manufactured by Ahlstrombased in FINLAND
Ahlstrom Sterile Barrier Systems (SBS) for sequential wrapping are designed to protect a wide variety of medical devices for optimum patient safety. Ahlstrom’s sterile barrier systems are a trusted and integral part of hospital CSSD. Focused on helping our customers stay ahead, Ahlstrom now offers a ...
-
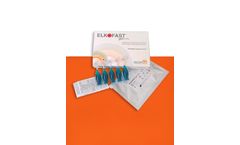
Manufactured by Molteni Therapeutics s.r.lbased in ITALY
ELKOFAST® gel 0.3% is a non sterile medical device (CE0373) topically administered recommended as an adjuvant for the local treatment of Skin lesions and ulcers through Photodynamic Therapy. The device is used in combination with a LED red light source at 630 ...
-
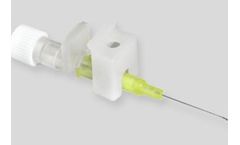
Manufactured by JCM MEDbased in FRANCE
IV cannulas with wing holder / cover, specially designed for one-handed insertion. IV cannulas with wing holder / cover, specially designed for one-handed insertion. The device carries small wings, specially designed for easy gripping and safe clamping to allow secure fixation to ...
-
Manufactured by Jinan Olabo Technology Co., Ltdbased in CHINA
Support the use of disinfectants such as hydrogen peroxide, sodium hypochlorite, and peracetic ...
-
Manufactured by Sterimedbased in FRANCE
This top web solution made of more than 90% of renewable resources is also fully compliant with the ISO 11607-1 and EN868-7 standards and specifically designed for EO and Irradiation sterilization ...
-
Manufactured by JETEMA, Co., Ltd.based in SOUTH KOREA
Product name : Graft/prosthesis, biomaterial; Product Authorization Number : 17-203; Storage : 2°C ~ 25°C (Do not freeze); Indication : Please refer to the instruction for use; [Single use / Sterile medical devices / Do not ...
-
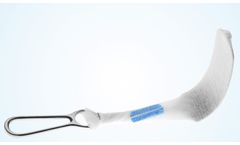
Manufactured by ASANUS Medizintechnik GmbHbased in GERMANY
The ASANUS retractor covers STERI COVER have been proving their worth in everyday operating theatres for years - following recertification, they are now available with IIA approval in accordance with Annex II of Directive 93/42/EEC for medical devices. The sterile single use retractor covers from ASANUS offer an easy and quick application due to the secure velcro ...
-
Manufactured by Ahlstrombased in FINLAND
Ahlstrom’s effective bacterial-barrier paper for packaging sterilized medical devices is sealable, peelable and ...
-
Manufactured by JETEMA, Co., Ltd.based in SOUTH KOREA
Product Name : Polydioxanone suture; Model Name : epiticon; Product Authorization Number : 15-1636; Purpose of use : It is contouring lifting thread, general-purpose catheter cannula, and sterilized needle. It is used for the purpose of medical treatment for the purpose of not administering drugs and the like.; [Single use / Sterile ...
-
Manufactured by JETEMA, Co., Ltd.based in SOUTH KOREA
Model Name : epiticon JAMBER. Product Authorization Number : No.13-1507 Purpose : Comprised of polydioxanone suture, catheter, and sterilized needle; used for treatment of human body, but not for drug delivery. Disposable/sterilized medical device/do not ...
-
Manufactured by JETEMA, Co., Ltd.based in SOUTH KOREA
Product name : epiticon Thin, polydioxanone suture; Model name : epiticon Thin; Product approval number : No.13-1039; Purpose : Comprised of polydioxanone suture, catheter, and sterilized needle; used for treatment of human body, but not for drug delivery; [Disposable/sterilized medical device/do not ...
-
Manufactured by Meridian Medicalbased in UNITED KINGDOM
Our medical equipment manufacturing facilities include 9,000 square feet of clean rooms that offer a mix of ISO class 7 and 8 purpose-built production ...
-
Manufactured by OPIA Technologiesbased in FRANCE
EYEPRIM is a sterile medical device designed to perform conjunctival impressions of the living eye. It enables further analysis of cells and biomarkers to help diagnose several ocular surface disorders, such as dry eye, allergies or infections. The EYEPRIM device is based on an original idea, suggested by Christophe Baudouin, MD, PhD, Chairman of Department of ...
-
Manufactured by HAGMEDbased in POLAND
Electrophysiological electrode extension cables are used to connect electrophysiological electrodes (ablation electrodes, diagnostic electrodes for cardiac electrophysiology and electrodes for temporary cardiac pacing) to electrophysiological medical devices, to transfer radio frequency current from medical electrophysiological devices (ablators) to the heart, or ...
-
Manufactured by JCM MEDbased in FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. These IV cannulas are fitted with an injection port that allows the supplementation of a drug into the cannula itself by a syringe without a ...
-
Manufactured by Prodimedbased in FRANCE
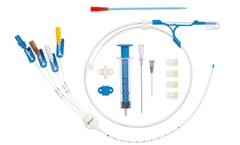
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
-
Manufactured by JCM MEDbased in FRANCE
JCM MED Intravenous Cannulas / Catheters allow the introduction or withdrawal of fluids from the human circulatory system. The short flexible and kink-resistant catheter is introduced into a blood vessel over a hollow introducer needle. Also called « one-way » IV cannulas, these cannulas may be supplied, in their sophiticated version, with hydrophobic filters to avoid ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you