Interventional Procedure Equipment & Supplies
-
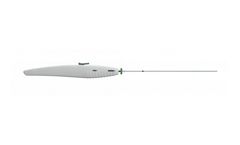
Manufactured by SentreHEART, Inc.based in USA
The FindrWIRZ Guide Wire System provides an innovative approach to diagnostic and interventional procedures where control of placement and delivery is ...
-
Manufactured by Blue Neem Medical Devices Pvt Ltdbased in INDIA
Used for dilation of the ureter through natural orifice prior to a diagnostic or interventional procedure ...
-
Manufactured by Galt Medical Corp.based in USA
Galt Medical’s Micro-Access Hemostasis Valve Introducer Kits provide the high quality expected for use during Cardiac Cath Lab and interventional procedures. They are specially designed to provide consistent performance every time. Kink resistant material. Smooth transition between dilator and sheath. .035" and .038" configurations are ...
-
Manufactured by Blue Neem Medical Devices Pvt Ltdbased in INDIA
Used to gain access to desired anatomical location and to assist in placement of medical devices during diagnostic or interventional procedures. Nitinol core with blue & white teflon sleeve, designed to provide excellent ...
-
Manufactured by Transluminal Technologies, LLCbased in USA
A first-of-its-kind magnesium alloy-based vascular closure device that offers a unique method of quickly achieving stable mechanical vascular closure following percutaneous catheterization for diagnostic or interventional procedures using 5-8 Fr procedural sheaths. The implant consists of a plug and footplate system that when presented to the ...
-
Manufactured by Garwood Medical Devices, LLCbased in USA
Currently under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures while maintaining the current standard of ...
-
Manufactured by APT Medicalbased in CHINA
Braidin™ L guiding sheath inherits the premium quality as APT Medical hemostasis introducer, features braided structure and marked tip to provide superior torqueability and back-up support, it is wildly used in peripheral intervention and venous ...
-
Manufactured by Precision Coating Company, Incbased in USA
Typical devices coated in interventional cardiology procedures are guide wires and core wires. PTFE provides low friction to a wire allowing for ease of insertion of a catheter and high release (non-stick) to prevent blood from clotting on the wire. Wire sizes range from 0.013” to 0.022” diameter with coating tolerances from 0.0002” to ...
-
Manufactured by CIVCO Medical Solutionsbased in USA
Envision ultrasound probe covers and scanning pads are activated with a sterile liquid and require no gel. Envision ultrasound viral barriers benefit clinicians and patients during interventions and procedures utilizing ultrasound, such ...
-
Manufactured by IMS Giotto S.p.Abased in ITALY
Featuring a unique and ergonomic design to ensure patient comfort and easiness for the operator: the gantry is able to tilt and rotate down 90° for interventional procedures with the patient in prone ...
-
Manufactured by Fonar Corporationbased in USA
Since the FONAR 360° has ample room for an entire surgical team and its equipment, and allows for unrestricted 360-degreee access to the patient, the scanner can be fully outfitted for MRI-guided surgical and interventional procedures. Equipped for this future application, the scanner is called the OR-360° (MRI Operating Room). The OR-360°™ (MRI ...
-
Manufactured by Italraybased in ITALY
Powerful fluoroscopy mobile c arm units designed for a wide range of applications: from orthopedic surgery (RX control of prosthesis positioning, plate installation), to general surgery (intraoperative control), Cardiac surgery (Pace-Maker implant, electrophysiology), therapy of pain, endoscopy and interventional ...
-
Manufactured by Cordisbased in USA
The EXOSEAL VCD is indicated for femoral artery puncture site closure, reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional catheterization procedures using a standard 5F, 6F, or 7F vascular sheath introducer with up to a 12-cm working ...
-
Manufactured by Innovative Medical Mannheim GmbH IMMbased in GERMANY
Robotic Emulator Laparoscopic Maryland dissector of only 5mm incisive diameter, with the exclusive novelty of articulated incisal head with 360 degrees of rotation, the articulated head emulating the robotic movements, is a great convenience and unavailable in the market today in such diameter, robot like movements with smaller incision will reduce the negative impact of the ...
-
Manufactured by Innovative Medical Mannheim GmbH IMMbased in GERMANY
Robotic Emulator Laparoscopic Grasper of only 5mm incisive diameter, with the exclusive novelty of articulated incisal head with 360 degrees of rotation, the articulated head emulating the robotic movements, is a great convenience and unavailable in the market today in such diameter, robot like movements with smaller incision will reduce the negative impact of the ...
-
Manufactured by Amsel Medical Corporationbased in USA
CE mark and FDA cleared SCureClamp with Interdigitation Occlusion Technology (iDOT) is designed to easily and securely close vessels – veins, arteries, and tubular structures – during surgical and interventional procedures. This unique mechanical surgical clamping technology is delivered directly through a fine, minimally invasively, 18 G needle for ...
-
Manufactured by Image Diagnostics, Inc. (IDI)based in USA
The Aspect ISR G3 is the flagship of the Aspect™ Series table line; designed to be the preferred imaging solution during interventional and vascular procedures. Featuring true iso-centric lateral roll – anatomy on the iso axis remains centered in the fluoroscopic field of ...
-
Manufactured by Innovative Medical Mannheim GmbH IMMbased in GERMANY
Robotic Emulator Laparoscopic Needle Holder (Under development) of only 5mm incisive diameter, with the exclusive novelty of articulated incisal head with 360 degrees of rotation, the articulated head emulating the robotic movements, is a great convenience and unavailable in the market today in such diameter, robot like movements with smaller incision will reduce the negative impact of the ...
-
Manufactured by Precision Coating Company, Incbased in USA
Precision Coating applies medical grade PTFE to devices used in interventional cardiology procedures focused on catheter-based treatment of structural heart diseases. Catheterization involves the insertion of a sheath into a peripheral artery or vein and cannulating the heart under X-ray visualization. A typical procedure would involve the ...
-
Manufactured by LVD Biotechbased in SPAIN
It enhances the surface lubricity and reduces possible tissue trauma. Hydrax® technology is primarily designed to enhance interventional procedures in coronary, peripheral, and neurovascular therapies, due to excellent lubricity and low friction properties. Hydrax® is the result of more than 10 years of research and development in the hydrophilic coating ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you