Infection Status Equipment & Supplies
-
based in NORWAY
PatoGen BioSafety Program™ is a comprehensive system for minimising the risk of disease and loss throughout the production cycle. PatoGen's aim is for the program both to prevent the introduction of infection as a result of a lack of knowledge of infection status in individual populations and enable potential disease problems to be ...
-
Manufactured by America Diagnostics, Inc.based in USA
This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
-
Manufactured by Abingdon Health (Medical)based in UNITED KINGDOM
Keep your employees in the know of their current COVID-19 infection status. The non-invasive COVID-19 lateral flow test is easy to use and ideal for self testing and will provide a result in less than 15 minutes. The test determines whether a person has a current COVID-19 infection and will enable appropriate decisions to be made about self ...
-
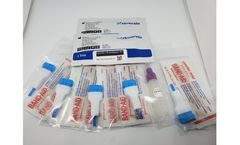
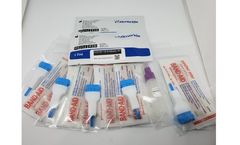
Manufactured by America Diagnostics, Inc.based in USA
This test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection ...
-
Manufactured by Access Biobased in USA
Qualitatively detect the SARS-CoV-2 nucleocapsid protein. Use for nasal swab specimen. Fast results only in 10 minutes. Identify individual’s current infection status to ...
-
based in TAIWAN
Ampholipad is a generic of AmBisome, a lipid-encapsulated version of the antifungal drug amphotericin B. Ampholipad successfully demonstrated bioequivalence to AmBisome in all three forms and in both large and small batches, making it the first and only drug to have achieved such a feat. Scaled-up production of Ampholipad™ can surpass a million vials each year, at a capacity capable of ...
-
Manufactured by Auro Vaccines LLCbased in USA
Jackson Foundation, Auro Vaccines is developing a subunit vaccine (HeV-sG-V) composed of the envelope glycoprotein of Hendra virus for the prevention of Nipah virus (NiV) ...
-
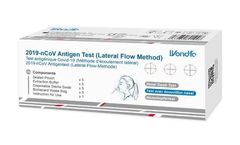
Manufactured by Abingdon Health (Medical)based in UNITED KINGDOM
Wondfo – COVID-19 Lateral Flow Antigen Test is a home self-test for detecting the SARS-CoV-2 virus. Detect SARS-COV-2 (COVID-19) virus in 15 minutes. No need to go to the shop – we’ll send the tests to you!. MHRA Registered. Pack Size: 5?Tests. Product Code: ...
-
Manufactured by DxTerity Diagnostics Inc.based in USA
COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. Older adults and people of any age who have underlying medical conditions have a higher risk of severe illness from COVID-19. Serious ...
-
Manufactured by Co-Diagnostics, Inc.based in USA
The Logix Smart™ ABC (Influenza A/B, SARS-CoV-2) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of the RNA from the Influenza A, Influenza B, and SARS-CoV-2 viruses. The multiplex test operates using a single step real-time reverse transcriptase polymerase chain reaction (RT-PCR) process in lower respiratory tract ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you