In Vivo Model Equipment & Supplies
-
based in
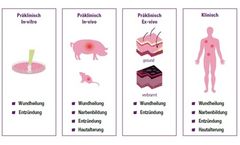
Clinical models enable realistic investigations of wound healing and are crucial for the development of new therapies. We have specialised in the development and establishment of clinically relevant in-vivo models to better understand key processes in the skin (e.g. wound healing, scarring, skin ageing,...). ...
-
Manufactured by InnoSer Belgie NVbased in BELGIUM
Slow progression with cysts in all segments of the ...
-
Manufactured by Inovotion Sasbased in FRANCE
INOVOTION has developed a unique, highly sensitive and reproducible assay. It is applicable to all preclinical oncology and immuno-oncology drug discovery programs, and monitors the growth and metastatic dissemination of human tumor ...
-
Manufactured by Acuitas Therapeuticsbased in CANADA
Acuitas specializes in the development of lipid nanoparticle delivery systems for molecular therapeutics. Our scientists have synthesized over 500 novel cationic lipids which have been evaluated in in vivo models including non-human primate (NHP) models. This evaluation characterizes the potency and safety of these LNP carriers for delivery ...
-
Manufactured by Xtant Medicalbased in USA
The graft does not adhere to gloves yet maintains its placement in the surgical environment. Osteoinductivity of sterile final product is assessed in vivo. In this challenging model, every lot tested to date has consistently demonstrated an osteoinductive response. This performance validates Xtant Medical’s expertise in DBM processing. ...
-
based in USA
User Benefits: Geistlich Fibro-Gide® is a porcine, porous, resorbable and volume-stable collagen matrix, specifically designed for soft-tissue regeneration.1,4,10 The collagen matrix is made of reconstituted collagen and undergoes smart chemically cross-linking to improve its volume stability while maintaining a good biocompatibility.1-3,4-10 The porous network of Geistlich Fibro-Gide® ...
-
Manufactured by InSphero AGbased in SWITZERLAND
An in vitro PDX model with in vivo-like heterogeneity. 3D InSight™ Tumor Microtissues from Patient-Derived Xenografts (PDX) are custom assay-ready 3D in vitro tumor models engineered to reflect complex tumor biology without the use of artificial matrices. ...
-
Manufactured by Absolute Antibody Ltdbased in UNITED KINGDOM
Recombinant monoclonal antibody to Amphibian Allergen. Manufactured using AbAb’s Recombinant Platform with variable regions (i.e. specificity) from the hybridoma ...
-
based in
Our human ex-vivo skin models are made from human skin samples and allow us researchers to study the physiological properties and functions of human skin in more detail. These models include different skin layers such as epidermis, dermis and subcutis and provide a fully intact three-dimensional skin architecture. ...
-
Manufactured by Auris Medical AGbased in SWITZERLAND
The OligoPhore™ / SemaPhore™ technology has been extensively and successfully tested with various siRNA and mRNA sequences in numerous disease models in mice. For the development of our first proprietary drug product, AM-401 (OligoPhore™ siRNA targeting KRAS), we have initiated a program of IND-enabling ...
-
based in USA
Our lead compound, known as OLX07010, is a small molecule inhibitor of tau self-association which has demonstrated in vivo efficacy in two transgenic mouse models of tauopathy, htau and JNPL3, representing tau aggregation in AD and inherited tauopathies, respectively. ...
-
by Cellurisbased in BRAZIL
The Celluris’ novel solution, named RfuCAR, was engineering in order to respond some challenges addressed by clinical trials around the world. RfuCAR is a bispecific in tandem receptor CAR, that is composed by an antigen binding domain and another domain that binds to an extracellular molecule from tumoral microenvironment. ...
-
based in CANADA
Zucara Therapeutics is developing ZT-01, a first-in-class, once-daily therapeutic to prevent insulin-induced hypoglycemia in patients using insulin therapy. ZT-01 is designed to inhibit somatostatin, a pancreatic hormone that impairs the glucagon response to hypoglycemia in people with insulin dependent diabetes. ZT-01 restores glucagon secretion to prevent hypoglycemia, which could dramatically ...
-
Manufactured by Emulatebased in USA
Study drug absorption and drug-drug interactions in a primary organoid-based model of the human small ...
-
Manufactured by Destiny Pharma plcbased in UNITED KINGDOM
There are two XF-73 dermal programmes in development. One is being run by our China partner and shareholder – China Medical Systems Holdings Limited (CMS) – focused on superficial skin infections and the other is an in-house programme looking at the use of XF-73 for the prevention and treatment of serious infections associated with burns and open wounds such as diabetic foot ulcers ...
-
Manufactured by Elpisciencebased in CHINA
ES014 is a first-in-class anti-CD39xTGF-β bispecific antibody (bsAb) that simultaneously targets the adenosine and TGF-β pathways, two major immunosuppressive mechanisms in the tumor microenvironment (TME). Solid tumors frequently express TGF-β, which suppresses T cell activation and induces CD39 expression, the rate-limiting enzyme in the ATP-adenosine pathway. The anti-CD39 ...
-
Manufactured by Defymedbased in FRANCE
A medical device for the physiological delivery of insulin, which is currently under development. Should make it possible to instantly administer an intraperitoneal (IP) injection of insulin using a simple subcutaneous injection (via an external connection to a syringe, pen, insulin pump, etc.). A biocompatible, non-biodegradable membrane that is permeable to insulin, but impermeable to the ...
-
Manufactured by Defymedbased in FRANCE
A medical device for the encapsulation of insulin-secreting cells, under development for the treatment of type 1 diabetes. Possibility of replacing the cells without the need for surgery, thanks to an input and output connection to the device. Use of non-biodegradable, biocompatible membranes with selective permeability: the glucose and insulin pass freely through the membrane while the immune ...
-
Manufactured by Yeasen Biotechnology (Shanghai) Co.,Ltd.based in CHINA
Yeasen provides diverse kinds of Ceturegel matrix based on different applications, such as cell morphology, biochemical function, migration, Invasion, and gene expression studies. Ceturegel Matrix LDEV-Free provided by Yeasen is suitable for 2D or 3D culture, in vivo tumorigenesis, and invasion and migration ...
-
Manufactured by Prellis Biologics, Inc.based in USA
EXIS Human Immune Response: Prellis utilizes its proprietary holographic printing technology to engineer 3D printed lymph node organoids (LNOs) that recreates the human immunobiology in vitro allowing direct access to a fully functional human immune system. EXIS is a powerful and scalable platform with applications in antibody discovery (human and llama), immunogenicity evaluation, and in vitro ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you