Implant Insertion Equipment & Supplies
-
Manufactured by Artman Instrumentsbased in USA
Introducing Artman's 50-Piece Dental Implant Placement and Removal Tools Kit – the comprehensive solution for successful implant procedures. With a complete range of essential tools, this kit provides dentists with everything they need for precise and efficient implant placement and ...
-
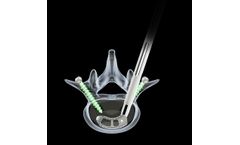
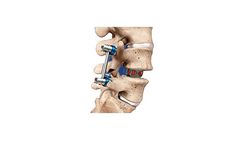
Manufactured by Biedermann Motech GmbH & Co. KGbased in GERMANY
implant and inserter. Bullet-nose design allows simplified entering. Angulation function supports exact positioning. Radiographic groove for lateral view and openings for A/P view. Lengths 28mm I 32mm I 36mm. Heights from 7 to 17mm. Parallel and 5° lordotic ...
-
Manufactured by Camber Spine Technologies, LLCbased in USA
The implant enables easy insertion with a smooth leading edge and a pocket that interlocks with the inserter to provide rotational stability. Combined with the arched design, newly-forming bone grows onto the superior and inferior surfaces of the implant, developing into a “mechanical fusion” between adjacent ...
-
Manufactured by LZQ Tool Co., Ltd.based in CHINA
Stainless metal - To select up and insert implants. They are used assembled with wrench, on hand in a number of sizes. Screwdriver prosthetic in a number of lengths is for slim straight and angulated conical abutments. (Short/Medium/Long). Included in the Prosthetic ...
-
Manufactured by Keystone Dentalbased in USA
K-LEAN™: Sandblasted acid-etched surface, with an extensive multi-stage cleaning process, utilizes ultra-pure water (UPW) which removes undesired residues, providing a clean surface and maintaining an intact oxide layer. ROUNDED APEX: The rounded apex protects the sinus floor and/or adjacent anatomical structures during implant insertion. ...
-
Manufactured by Keystone Dentalbased in USA
K-LEAN™: Sandblasted acid-etched surface, with an extensive multi-stage cleaning process, utilizes ultra-pure water (UPW) which removes undesired residues, providing a clean surface and maintaining an intact oxide layer. ROUNDED APEX: The rounded apex protects the sinus floor and/or adjacent anatomical structures during implant insertion. ...
-
Manufactured by Dieter Marquardt Medizintechnik GmbHbased in GERMANY
PipTree – Arthrodesis of the proximal interphalangeal joint of the small toes. Implant for PIP joint fusion.The PipTree implant is an intramedullary force carrier, which enables a stable osteosynthesis of the toe in a physiological position. This easy-to-insert titanium implant fixes the toe in the desired flexion position. ...
-
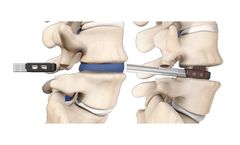
Manufactured by Spine Wave, Inc.based in USA
The Velocity Expandable Interbody Device is a composite, expandable implant that is designed for ease of insertion, optimal implant to patient fit, maximized indirect decompression, and maximum pre and post bone ...
-
Manufactured by Osseusbased in USA
The Blue Topaz Sacroiliac Screw System consists of fenestrated, double helix threaded screws with aggressive distal cutting flutes facilitating effortless bone purchase and screw insertion. The implant’s proprietary surface treatment and open architecture promote bone growth onto, into, and throughout the device. The screws come in a wide range of diameters ...
-
Manufactured by Bien-Air Dental SAbased in SWITZERLAND
The jet of physiological liquid is projected onto the drill shaft itself,providing perfect cooling, particularly when using implant guides for insertions. The CA 20:1 has a miniature head and is the lightest on the market. In addition, its shafts and gears are machined in stainless steel with high resistance to saline solutions. ...
-
Manufactured by Field Orthopaedicsbased in AUSTRALIA
The management of small fractures in the hand and wrist is difficult due to the small size, fragile nature, and importance of accurate reduction. The requirement to use clamps and forceps while maintaining position for implant preparation and insertion is challenging. The fully cannulated Micro Screw system is designed to address these challenges and make the ...
-
Manufactured by Hi-Tec Implantsbased in ISRAEL
Torque Wrench – adjustable from 10 Ncm to 45 Ncm Full ratchet position. 30 Ncm is used for the accurate tightening of most prosthetic components to correct torque, minimizing the risk of screw loosening. ...
-
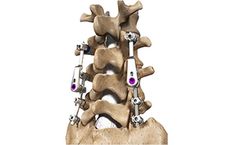
Manufactured by Spine Wave, Inc.based in USA
Secure to existing 5.5 mm diameter rod systems, including the CapSure® or Sniper® Spine ...
-
Manufactured by Anika Therapeutics, Inc.based in USA
The SpiralUp® TCL Allograft System is an acellular dermal allograft intended to supplement the talocalcaneal ligament and as such, function as a dense, stable and spongey connective tissue layer. It is intended to improve the function of the foot, as well as conditions along the musculoskeletal (MSK) chain. ...
-
Manufactured by Spine Wave, Inc.based in USA
The Leva® PX interbody device is an expandable titanium implant optimized for ease of insertion and maximum bone ...
-
Manufactured by Spine Wave, Inc.based in USA
Flexible Offering, Calibrated Design. The Abacus® Lateral Spacer System is available in multiple sizes with a biconvex implant shape designed to provide conformance to patient anatomy/pathology. The Abacus® Lateral Spacer System is intended to be introduced to the disc space via the lateral ...
-
Manufactured by Pinnacle Spine Groupbased in USA
InFill Interbody Fusion Systems patented in situ graft delivery solution represents an evolution in addressing the biologic void in spine ...
-
Manufactured by NuVasive, Inc.based in USA
X360 is a comprehensive lateral approach to single-position surgery that leverages advanced techniques and technologies to deliver patient-specific care while enhancing OR workflow and ...
-
Manufactured by Arrowhead Medical Device Technologies, LLCbased in USA
Commit to trying ARROW-LOK™ on at least five patients while closely following the recommended technique. As simple as the ARROW-LOK™ is and uncomplicated the operative technique may be, please expect to take at least five cases to become comfortable with the product, the operative technique and how the design interacts with the anatomy. Five (5) cases will give the surgeon the ...
-
Manufactured by Crossroads Extremity Systemsbased in USA
There are potential risks associated with the use of these devices, some of which include: allergic reaction to the implant material, fracture of the implant, soft-tissue complication (e.g., infection at the implant site, prolonged healing), and revision surgery. Refer to IFU for all contraindications, warnings, and risks. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you