COVID Kit Equipment & Supplies
-
Manufactured by Path-Tec, LLCbased in USA
Outer box; Outer box label; Medium Prefill; Swab; UN3373 Label on box; 95kPa Biohazard Bag; 50ml Absorbent; Patient Label; UN3373 LabPak; Lot Number ...
-
Manufactured by DxTerity Diagnostics Inc.based in USA
Our SARS-CoV-2 RT-PCR saliva-based test is FDA EUA authorized for at home unsupervised sample collection, making easy for you to take your test in the comfort of your home. Collect your sample at home and then ship it back to our lab. We use state-of-the-art technology to get your accurate results. Results are reported to you through our secure portal. ...
-
Manufactured by Access Biobased in USA
The CareStart™ COVID-19 MDx RT-PCR is a real-time reverse transcription polymerase chain reaction (RT-PCR) test intended for the qualitative detection of nucleic acid from SARS-CoV-2 in respiratory specimens (such as nasopharyngeal, oropharyngeal and nasal swabs, and nasopharyngeal wash/aspirate or nasal aspirate) and bronchoalveolar lavage from individuals suspected of ...
-
Manufactured by DxTerity Diagnostics Inc.based in USA
COVID-19 is caused by the SARS-CoV-2 virus which is a new virus in humans causing a contagious respiratory illness. COVID-19 can present with a mild to severe illness, although some people infected with COVID-19 may have no symptoms at all. ...
-
Manufactured by Bio-Diagnostics Ltd.based in UNITED KINGDOM
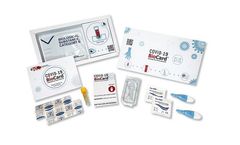
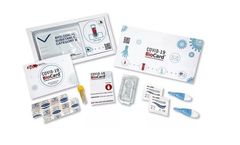
Antibody tests for Covid-19 – Each kit contains everything needed to test one person for specific COVID-19 ...
-
Manufactured by Bio-Diagnostics Ltd.based in UNITED KINGDOM
Antibody tests for Covid-19. Each kit contains everything needed to test one person for specific COVID-19 Antibodies. The BioCard is confirmed to detect spike antibodies made in response to successful ...
-
Manufactured by Fluxergybased in USA
Learn more about Fluxergy’s qualitative real-time reverse transcription polymerase chain reaction (RT-qPCR) test. For Research Use Only (RUO). Not for use in diagnostic ...
-
Manufactured by Fluxergybased in USA
Potential environments for rapid testing applications include emergency rooms, outpatient procedures, urgent care, workplace screening, mobile testing, community level testing settings, school reopenings, and tourism. Fluxergy’s easy-to-use qualitative RT-qPCR test can detect SARS-CoV-2 in one hour. Approved for CE-IVD. All other regions are for research use only. This device is ...
-
Manufactured by Shanghai Fosun Long March Medical Science Co., Ltdbased in CHINA
Features: Easy to use. Affordable. Highly portable, requiring no instrumentation. Reliable, with 95.2% sensitivity and 99.6% specificity. Fast, with results in 15 ...
-
Manufactured by Convergent Technologies GmbH & Co. KGbased in GERMANY
Furthermore, in many cases the manifestation of symptoms is very weak. Hence, proper diagnosis of COVID-19 can be a challenge. The Convergys® POC RT-PCR COVID-19 Detection Kit directly detects SARS-CoV-2 genetic material. ...
-
Manufactured by Trivitron Healthcarebased in INDIA
Immediate on-site Covid 19 Antigen Testing. BIOCARD Pro COVID-19 Rapid Antigen kit is a chromatographic immunoassay for the qualitative detection of specific antigens to COVID-19 present in human nasopharyngeal ...
-
Manufactured by Visby Medical, Inc.based in USA
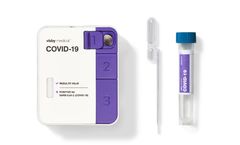
Fast, accurate, and actionable PCR results in under 30 minutes. Instrument-free PCR: Empower on-demand testing, when and where you need ...
-
Manufactured by NanoEnTek, Inc.based in SOUTH KOREA
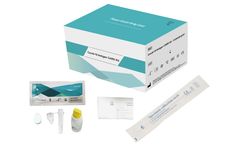
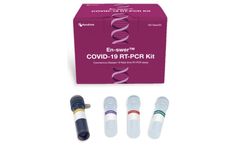
The En-swer™ COVID-19 RT-PCR Kit detects E, N, and RNaseP genes of SARS-CoV-2 qualitatively through real-time reverse-transcription polymerase chain reaction. It can be used for rapid detection and outbreak control of COVID-19. ...
-
Manufactured by LabTurbo Biotech Corporationbased in TAIWAN
LabTurbo™ AIO COVID-19 RNA Testing Kit is a multiplex real-time reverse transcription polymerase chain reaction, (RT-PCR) diagnostic product for SARS-CoV-2 RNA (COVID-19) detection. The multiplex reaction detects COVID-19 N1 gene, coronavirus E gene, and RNase P gene (internal control) in one well. ...
-
Manufactured by SD Biosensorbased in SOUTH KOREA
STANDARD F COVID-19 Ag FIA is the fl uorescent immunoassay for the qualitative detection of specific nucleoprotein antigens to SARS-CoV-2 present in human nasopharynx. STANDARD F COVID-19 Ag FIA should be used with the STANDARD F Analyzers manufactured by SD BIOSENSOR. ...
-
Manufactured by Talis Biomedical Corporationbased in USA
A front-line choice for quick, high volume SARS-CoV-2 antigen testing. Testing for COVID-19 is in high demand. SARS-CoV-2 antigen testing can be an alternative first line of defense when compared to PCR testing, particularly for symptomatic patients within a specific window from symptom onset and where tests are repeated frequently (i.e., weekly ...
-
Manufactured by Innovatek Medical Inc.based in CANADA
Virus specimen collection and transport kits. Health Canada approved and M-40 ...
-
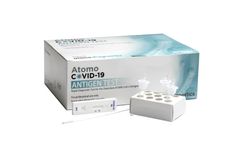
Manufactured by Atomo Diagnostics Limitedbased in AUSTRALIA
A fast, accurate point of care COVID-19 antigen test for the qualitative detection of nucleocapsid protein antigens specific to the SARS-CoV-2 virus. Utilising an anterior nasal swab specimen obtained from individuals suspected of having COVID-19, the Atomo COVID-19 Antigen Test is easy to use, giving a result in as little as 10 ...
-
Manufactured by DxTerity Diagnostics Inc.based in USA
The DxTerity SARS-CoV-2 (COVID-19) RT-PCR CE saliva-based test provides the convenience of at-home sample collection with results available within 24-72 hours. Our highly-sensitive, gold-standard PCR test has been verified to work with all COVID-19 ...
-
Distributed by Alban Scientific, Inc.based in USA
QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple. The test is authorized for home use with self-collected anterior nasal (nares) swab samples in individuals aged 2 and older. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you