Clinical Trial Equipment & Supplies
-
Manufactured by Exscientiabased in UNITED KINGDOM
In the landmark study, it was demonstrated for the first time that our functional precision-oncology platform can improve patient outcome in a prospective interventional ...
-
Manufactured by CanaQuest Medical Corp.based in CANADA
What are clinical trials? Clinical trials are a way to test new methods of diagnosing, treating, or preventing health conditions. The goal is to determine whether something is both safe and effective. A variety of things are evaluated through clinical trials, including: Effectiveness of ...
-
Manufactured by CanaQuest Medical Corp.based in CANADA
Clinical testing on humans can only begin after a pre-clinical phase, involving laboratory studies (in vitro) and tests on animals, which has shown that the experimental drug is considered safe and effective. However, no animal is sufficiently similar to humans (even genetically modified ones) to make human testing unnecessary. ...
-
Manufactured by Keystone Folding Box Co.based in USA
In terms of requirements and restrictions, few industries are as demanding as the world of clinical trials. We have dedicated years to developing breakthrough packaging solutions that have become widely accepted by some of the most respected names in the pharmaceutical ...
-
based in USA
The Curavit DCT Platform is the digital manifestation of our decentralized clinical trial services. From operations, through data collection, to patient experience, our proprietary platform manages every aspect of your trial and provides insight into your data. Our platform, reflects our expert processes, is built from industry-leading ...
-
Manufactured by MGC Diagnostics Corporationbased in USA
The MICRO 6000 is an economical choice for basic spirometry, clinical trials or simple office spirometry. COMPLETE BASIC SPIROMETRY: Including Forced Vital Capacity, Slow Vital Capacity, Maximum Voluntary Ventilation and Minute Tidal Ventilation including bronchochallenge testing ...
-
Manufactured by CanaQuest Medical Corp.based in CANADA
Mentanine®, supported by (cannabidiol “CBD” + IP formula) – molecules bond and synergistically attach to PPAR receptors allowing the formula to cross the blood-brain barrier (BBB) to target the central nervous system (the brain) with amplified effects and efficacy. CBD by itself did not do this in the pre-clinical trials. ...
-
Manufactured by Rebiotix Inc., a Ferring Companybased in USA
Rebiotix Inc., a Ferring Company, has leveraged its clinical, manufacturing and regulatory experience with RBX2660 to develop the first of its kind non-frozen, room temperature stable oral microbiota-based formulation under the MRT™ drug platform, known as RBX7455. While it is currently used to reduce the incidence of recurrent Clostridioides difficile infection, additional ...
-
Manufactured by PsiOxus Therapeutics Ltd.based in UNITED KINGDOM
Novel transgene combinations. Our research team are developing a suite of viral vectors with new and innovative transgene ...
-
Manufactured by PsiOxus Therapeutics Ltd.based in UNITED KINGDOM
Two transgenes: Producing a secreted CD40 agonist monoclonal antibody. Currently in clinical trials in the US and UK. ...
-
Manufactured by Rubius Therapeutics, Inc.based in USA
Study Title: Open-label, multicenter, multidose, first-in-human, Phase 1/2 study of RTX-224 for the treatment of patients with relapsed or refractory, or locally advanced solid tumors, including non-small cell lung cancer, cutaneous melanoma, head and neck squamous cell carcinoma, urothelial (bladder) carcinoma and triple-negative breast cancer. Status: ...
-
Manufactured by Rubius Therapeutics, Inc.based in USA
Study Title: Open label, multicenter, multidose, first-in-human Phase 1 study of RTX-240 in combination with pembrolizumab for the treatment of advanced solid tumors. Status: ...
-
Manufactured by PsiOxus Therapeutics Ltd.based in UNITED KINGDOM
As part of our expanding T-SIGn platform pipeline, this candidate is progressing through its IND enabling activities in readiness for entering clinical ...
-
Manufactured by Rubius Therapeutics, Inc.based in USA
Study Title: Open label, multicenter, multidose, first-in-human Phase 1/2 study of RTX-240 for the treatment of patients with relapsed/refractory or locally advanced solid tumors. Status: ...
-
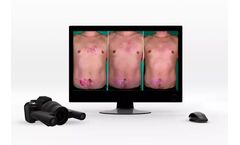
Manufactured by Quantificare Inc.based in USA
The 2D DermaViz® is a fully handheld photography device equipped with fixed optics and a built-in ranging light system, ensuring high-quality, reproducible images. Designed for clinical staff use, this system is easy-to-use and delivers consistent results regardless of the extent of previous experience in photography. ...
-
Manufactured by Quantificare Inc.based in USA
The 3D LifeViz® Series offers a range of high-quality 3D stereoscopic systems to consistently document any part of the body with unmatched accuracy and precision. All of our 3D systems are fully handheld and rely on a built-in ranging light system to ensure high-quality, reproducible images. Combined with our image analysis services, the 3D LifeViz® systems offer a powerful solution to ...
-
Manufactured by Transgenebased in FRANCE
A particularly innovative immunotherapy, TG4050 is the lead myvac® candidate. This individualized treatment is based on the specific mutations that are identified in each patient’s ...
-
Manufactured by Lifetech Scientific (Shenzhen) Co., Ltd.based in CHINA
Nitinol wire frame coated with Titanium Nitride (TiN). Decrease the dissolution of nickel ion efficiently, expected safe long-term biocompatibility. Promote the growth of endothelial tissue, lessen thrombus complication. Superior superelastic, effectively reduce atrioventricular block ...
-
by Genmab A/Sbased in DENMARK
DuoBody-CD40x4-1BB (GEN1042) is a proprietary bispecific antibody, jointly owned by Genmab and BioNTech, created using Genmab’s DuoBody® technology. It is being co-developed under an agreement in which the companies share all costs and future profits for the product on a 50:50 basis. CD40 and 4-1BB were selected as targets to enhance both dendritic cells (DC) and antigen-dependent ...
-
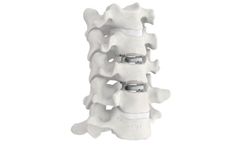
Manufactured by ZimVie Inc.based in USA
Mobi-C was determined by the FDA to be statistically superior to fusion at 7 years for two-level cervical disc replacement, based on the primary study endpoint of a prospective, concurrently controlled and randomized, multi-center clinical trial.1 At 10 years, all patient-reported outcomes were equivalent to or improved from 7 years.3.4 ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you