Class III Medical Device Equipment & Supplies
-
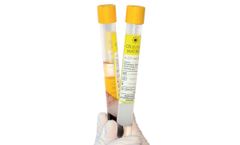
Manufactured by Regen Lab SAbased in SWITZERLAND
Cellular Matrix® tubes allow the preparation of autologous platelet-rich plasma (RegenPRP®) combined with a non-crosslinked hyaluronic acid (HA) in a closed-circuit system. CM-PRP-HA has an excellent safety profile in clinical practice.1,3,4. Cellular Matrix® technology combines the complementary clinical effects of RegenPRP® and hyaluronic acid (HA), giving better and ...
-
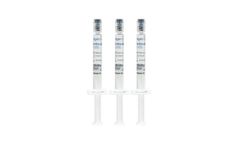
Manufactured by Regen Lab SAbased in SWITZERLAND
ArthroVisc 40 is a non-cross-linked hyaluronic acid (2 %) obtained from bacterial fermentation. This product is sterile and non-pyrogenic. It is packaged in 2 ml syringes of hyaluronic acid (20 mg/ml, 1500 KDa). ArthroVisc 40 is available in three syringes of 2 ml. It is for single use and designed to be used with sterile needles (25G or 27G), not supplied in the kit. ...
-
Manufactured by Integer Holdings Corporationbased in USA
As a Class III Medical Device manufacturer, our OEM partners trust us to custom design, develop and manufacture their implantable pulse generators (IPG) and lead systems. We honor that trust with a focus on accelerating time to market and reducing program risk and commercialization costs. ...
-
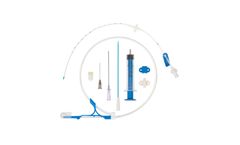
Manufactured by Prodimedbased in FRANCE
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
-
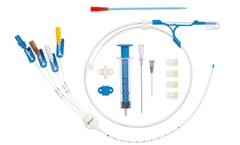
Manufactured by Prodimedbased in FRANCE
Central venous catheters for introduction by the Seldinger technique. Class III sterile medical ...
-
Manufactured by Vida S.r.l.based in ITALY
Our body filler is a CE certified III class medical device. Our revolutionary macromolecular advanced hyaluronic acid body filler 100% Made in Italy. It remodels the body in a totally safe way without surgery. It allows to harmonize the large volumes of the body such as glutes, calves, forearms and areas where a natural ...
-
Manufactured by Micro Crystal AGbased in SWITZERLAND
CC8A-T1A Medical is dedicated for specific use in Class III implantable medical devices and benefits from long-term reliability which is of utmost importance and a hallmark of Micro Crystal’s entire medical implantable grade portfolio. ...
-
Manufactured by Cirtec Medicalbased in USA
Long known for providing complete outsourcing solutions for Class II and III medical devices, Cirtec is pleased to announce we are now producing continuous draw Nitinol tubing. We understand that designing devices in a highly regulated industry can be challenging and our experienced and knowledgeable Nitinol team ...
-
Manufactured by Chemence Incbased in USA
Our company is a world leader in cyanoacrylate technology research and has developed a wide range of high-performance grades of industrial cyanoacrylate adhesives based on ethyl, methyl, methoxyethyl, isopropyl, n-butyl and octyl monomers over the years. Servicing three main industries – medical, industrial and consumer – Chemence® continues to push the ...
-
Manufactured by NextPhase Medical Devices LLC.based in MYANMAR
We understand the unique standards and requirements of Class II and III medical ...
-
Manufactured by Broncus Medical, Inc.based in USA
The Archimedes® Lite™ Virtual Bronchoscopic Navigation System, also known as LungPoint Plus® in Asia Pacific regions, is a simple, yet elegant virtual bronchoscopic navigation system that leverages the advanced navigation technology from Archimedes without fluoroscopy. Archimedes Lite is the only pulmonary navigation system that provides compatibility with any bronchoscope allowing ...
-
Manufactured by Cousin Surgerybased in FRANCE
4DVentral® is a partially resorbable and hydrophilic implant of intermediate density designed for the extraperitoneal treatment of ventral and incisional hernias. For the extraperitoneal treatment and reinforcement of ventral and incisional ...
-
Manufactured by Cousin Surgerybased in FRANCE
75% resorbable mesh: made of monofilament polypropylene and poly-L-lactic Acid (PLLA) knit. For the parietal treatment and reinforcement of inguinal and femoral ...
-
Manufactured by Cousin Surgerybased in FRANCE
Adhesix® Bioring® is an adjustable gastric band treating obesity by a mini-invasive and reversible surgical technique. This device is MRI compatible. An attestation is available on demand if necessary. You can ask for it via the contact page of the website. The Adhesix® Bioring® adjustable gastric band system is indicated for the reduction of excess body weight in ...
-
Manufactured by Cousin Surgerybased in FRANCE
Inflatable expansion balloon parietal prosthesis, knitted monofilament polypropylene and poly-L-lactic acid (PLLA). The implant is semi-resorbable. Repair and parietal reinforcement of umbilical, incisional hernias and small eventrations. Extra-peritoneal ...
-
based in FINLAND
Bonalive putty MIS is a smart bone regeneration technology, providing stable positioning and controlled access in minimally invasive ...
-
Manufactured by MBP – Medical Biomaterial Products GmbHbased in GERMANY
MB-Collagen is a collagen based dressing for wound care and traumatic, slowly healing wounds like Decubitus or leg ulcers (Ulcus cruris). The effectiveness of MB-Collagen is based on one hand on the chemotactic effects of the collagen matrix leading to a settlement of fibroblasts on the wound bed. On the other hand exogen collagen promotes binding of proteases. Both mechanisms are acting ...
-
Manufactured by MBP – Medical Biomaterial Products GmbHbased in GERMANY
Xenoguard is a cell-free collagen matrix derived from porcine pericardium. Xenoguard is intended for use as a surgical membrane for soft tissue repair, serving as a scaffold material and tissue coverage or tissue replacement for surgical applications where a longer lifetime of the implant after placement is requested. The double sterile packed membrane is available in various sizes. Xenoguard is ...
-
Manufactured by KAF GRUPbased in TURKEY
ONEGEL is a sterile, water-soluble lubricating gel used to lubricate catheters and other medical devices during urethral, colon and rectal applications such as urethral catheterization, endoscopy and cystoscopy. It can be also used for rectal and colonic applications as a lubricant gel. The most important function of the gel is to form a lubricating layer between ...
-
Manufactured by Meccellis Biotechbased in FRANCE
CELLIS® Dental is a cell-free, non-pyrogenic collagen matrix (acellular dermal matrix ADM) obtained from porcine skin. CELLIS® Dental is intended for use as a surgical membrane in soft tissue repairs and serves to support, cover or replace ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you