- Home
- Companies
- Dyve Biosciences
- Products
- Dyve - Breakthrough Transdermal ...
Dyve - Breakthrough Transdermal Technology
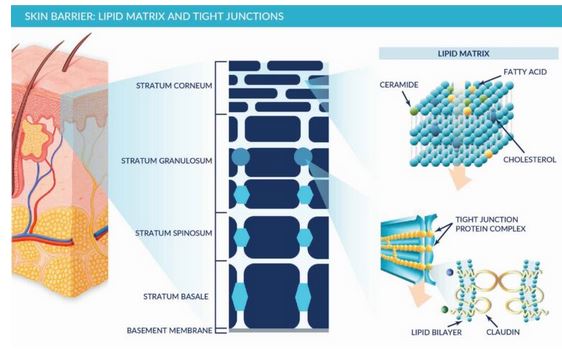
The skin is the body’s natural protective layer, with two primary barriers to passage: the stratum corneum on the surface and restrictive junctional proteins deeper in the skin. The stratum corneum is composed of flattened corneocytes set within a waxy, lipid matrix. This barrier is very effective at keeping water and nutrients inside the body and pathogens and chemicals out, making it the largest hurdle for most transdermal delivery technologies. The deeper junctional proteins further function to hold adjacent skin cells together and provide another barrier to transdermal drug delivery.
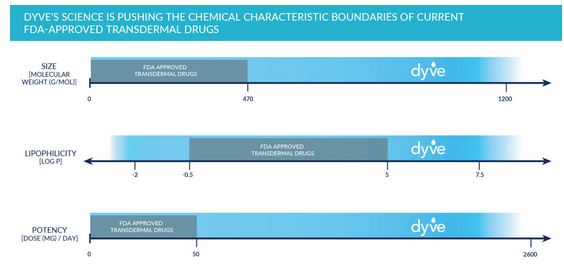
Of the 10,000+ drugs currently approved by the FDA, less than 30 are approved for transdermal delivery. This limited success has only been achieved with drugs that fall within very restricted chemical characteristic boundaries; they are low in molecular weight, moderately lipophilic, and highly potent.
Why is Dyve Different?
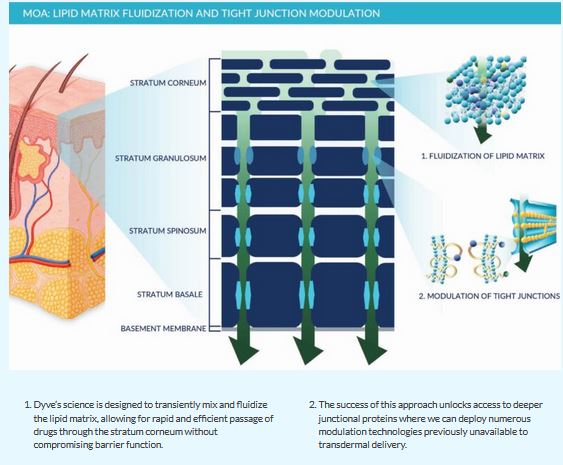
Current transdermal technologies often employ harsh solvents or complicated devices to break down the barriers of the skin resulting in skin irritation and damage with chronic use. Alternatively, Dyve has developed an elegant microemulsion, which transiently and reversibly fluidizes the lipid matrix and modulates tight junctions with topical application, allowing for non-destructive delivery through the skin and into the blood with uncompromised pharmacokinetics.
The Impact of Dyve’s Science
Dyve’s science shows the historic dogma of what can be delivered through the skin no longer applies. Successful delivery has been achieved with a wide range of molecules; the limit to what Dyve’s science can accomplish is yet to be defined.
Our proprietary science enables drug delivery with many of the pharmacokinetic advantages of both needles and pills, dramatically outperforming other topical options and creating clear clinical differentiation from the current standard of care.
- Safely delivering molecules with chemical characteristics well outside the limits of other transdermal technologies
- Achieving unprecedented bioavailability compared to other transdermal attempts
- Attaining systemic delivery that exceeds oral benchmarks while avoiding first-pass metabolism and GI-related adverse events
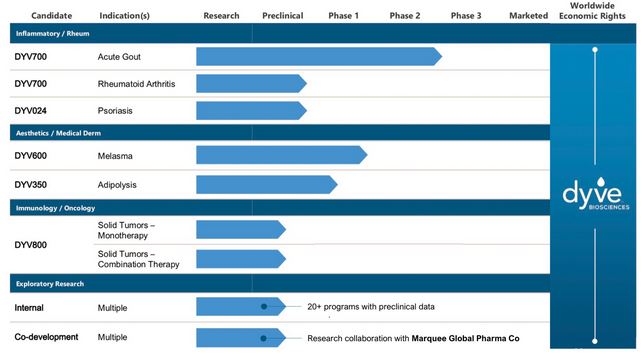
Dyve’s lead asset, DYV700, validates our platform technology and approach with existing POC data. In an initial randomized double blind placebo controlled trial, DYV700 provided patients experiencing gout flares with fast-acting relief that mitigated pain in minutes vs. days with the existing standard of care. Our pipeline includes Human POC data for 9 programs across three therapeutic areas that all utilize Dyve’s novel transdermal delivery approach.