Pre-Clinical Equipment Supplied In Illinois
-
Manufactured by Auris Medical AGbased in SWITZERLAND
The OligoPhore™ / SemaPhore™ technology has been extensively and successfully tested with various siRNA and mRNA sequences in numerous disease models in mice. For the development of our first proprietary drug product, AM-401 (OligoPhore™ siRNA targeting KRAS), we have initiated a program of IND-enabling ...
-
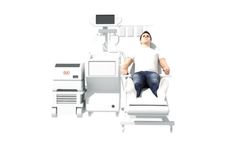
based in USA
Inhaled Imatinib for PAH: We have advanced inhaled imatinib, AER-901, into the clinic for the treatment of patients with pulmonary arterial hypertension (PAH). The Phase 1 trial is currently ...
-
Manufactured by Auris Medical AGbased in SWITZERLAND
AM-125 for the treatment of vertigo is expected to complete its Phase 2 TRAVERS trial in Q2 2022 and to move into Phase 3 in Q4 2022. Keyzilen® (AM-101) for the treatment of acute inner ear tinnitus and Sonsuvi® (AM-111) for the treatment of acute inner ear hearing loss have both been evaluated in Phase 3 trials, but will require further clinical testing. ...
-
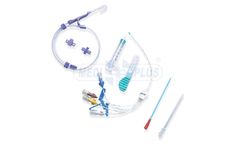
Manufactured by Shree Umiya Surgical Pvt. Ltd.based in INDIA
Central Venous Catheter made of specially formulated and biocompatible Polyurethane material provides strength during insertion and also softens at body temperature to conform to the body tissues and reduces the risk and vascular trauma. ...
-
Manufactured by Lupagen Inc.based in USA
Lupagen avoids the cost & complexity of cell therapy and the safety & clinical challenges of viral-based gene therapy by a simple patient-connected gene delivery procedure done at the bedside. Our Side CAR-T® delivery system, a first-in-class bedside system enables gene delivery directly to target cells in a highly-controlled and safe environment before reinfusing therapies to the ...
-
Manufactured by Centric Medical, LLCbased in USA
Osteo-Link has been engineered and processed to deliver the highest level of osteoinductivity, according to in-vivo test results. Differentiated putty, strips, cubes, and filler are available to help influence better patient outcomes and reduce costs. ...
-
Manufactured by Jointechlabs Inc.based in USA
MiniTC™ is an FDA-cleared portable medical device for isolation of fragmented and purified fat grafts (microfat) at the point-of-care. It is a patented, disposable, closed loop medical device that can be used for processing of lipoaspirate (fat) in any clinic setting, with no change in infrastructure. The end-product is finely washed injectable microfat which can be used for autologous ...
-
Manufactured by Jointechlabs Inc.based in USA
Mini-Stem System™ system is a CE-mark pending medical device for isolation of fat-derived stromal vascular fraction (SVF) at the point-of-care. It is a patented, disposable, closed loop medical device that can be used for processing of lipoaspirate (fat) in any clinic setting, with no change in infrastructure. The end-product is an injectable stem cell fraction (SVF) which can be used for ...
-
Manufactured by Jointechlabs Inc.based in USA
Based on our platform and technology, Jointechlabs is developing therapies for unmet medical needs. Our first therapeutic in clinical development JTL-T-01 is a proprietary injectable stem cell scaffold as a biologic therapy for osteoarthritis, for approval under the FDA’s fast-track program. JTL-T-01 has been designed as an autologous point-of-care therapy enabling the patient’s own ...
-
Manufactured by FaceLakebased in USA
The Facelake FL400 Pink Fingertip Pulse Oximeter quickly measures pulse rate and blood oxygen saturation levels in the body. The FL400 was designed for use by pilots and sports enthusiasts. Pulse rate/SpO2 readings are taken with a swift measurement from the user’s finger. The user’s finger is inserted into the padded interior of the device that was designed to comfortably accommodate ...
-
Manufactured by Brainlab AGbased in GERMANY
KingMark accurately calibrates AP pelvic images and measures patient ...
-
Manufactured by Avery Dennison Medicalbased in USA
The Absorbent Wound Dressing portfolio is indicated for the management of lightly to moderately exuding chronic and acute wounds. The range is designed to promote a moist wound environment, which can support healing. The portfolio includes products designed for partial and full thickness pressure sores and leg ulcers as well as superficial wounds, skin tears and postoperative ...
-
Manufactured by Bisco, Inc.based in USA
-
Manufactured by Merete Technologies, Inc. (MTI)based in USA
For correcting axis misalignment (Genu varum/valgum) using hemi-epiphysiodesis. The flexible center area of the anatomically shaped PediatrOS™ FlexTack™ allows it to bend open in vivo in response to bone growth forces, helping steer pediatric and adolescent bone growth gently and precisely. The trapezoidal design of the PediatrOS™ FlexTack™ staple is closely aligned to the ...
-
Manufactured by Shamrock Labelsbased in USA
Size: 1-1/2 " x 2". Unit of Measure: Roll. Qty per UOM: 500. Material: ...
-
Manufactured by Shamrock Labelsbased in USA
Size: 1" DIAMETER. Unit of Measure: Roll. Qty per UOM: 500. Material: Superstick. Adhesive: ...
-
based in USA
Why Inhaled Therapies: Delivery of therapeutics directly to the lungs allows for more precise doses of drugs to achieve therapeutic benefit and avoid adverse events seen with orals and injectables. For a number of cardiopulmonary and cardiometabolic conditions, treatment via inhalation is an ideal alternative to routine injections allowing for improved drug bioavailability and efficacy while ...
-
Manufactured by Life Spine, Inc.based in USA
Osteo-Link has been engineered and processed to deliver the highest level of osteoinductivity, according to in-vivo test results. Available in putty, strips, cubes, and flowable for a variety of ...
-
Manufactured by Baxter International Inc.based in USA
Floseal is an effective adjunct hemostatic agent proven in a wide-range of bleeding scenarios1 with a proprietary combination of two independent hemostatic ...
-
Manufactured by Unified Information Devices, Inc (UID)based in USA
The UID Implantable Programmable Microchip (UC-2112) is ideal for positive and permanent identification in rats and larger laboratory animals. With microchip dimensions of 2.1 mm in diameter and 12 mm in length, it is the is the most common size for use in almost all laboratory animals. Each microchip is supplied individually packaged in sterile cannula for quick and easy implantation using a ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you