Pre-Clinical Equipment Supplied In Spain
-
PremiumManufactured by Environics, Inc.based in USA
The Environics Series Reduced Oxygen Breathing Device 2 is the next generation system that simulates altitude exposure and can be utilized for both research and hypoxia ...
-
Manufactured by PHARMAQ AS - part of Zoetisbased in NORWAY
ALPHA JECT micro 1 PD is a monovalent vaccine developed for use in Atlantic salmon, Salmo salar L. It contains inactivated SAV 3 and is documented for use either alone, or simultaneously with PHARMAQ multivalent vaccines. ALPHA JECT micro 1 PD has been optimized through extensive research to reduce mortality, lesions in the heart and pancreas and impaired growth caused by PD. The efficacy of the ...
-
based in USA
Mucopolysaccharidosis type 1 (MPS 1) is a rare lysosomal storage disease that is caused by mutations in IDUA, the gene that encodes the alpha-L-iduronidase (IDUA) enzyme. These mutations cause the misfolding and dysfunction of IDUA, which leads to the toxic buildup of large sugars in the bone, cartilage, cornea, heart and central nervous system (CNS). There is currently no cure for these ...
-
Manufactured by Nanovex Biotechnologiesbased in SPAIN
Fluorescent nanovesicles are very useful to in vivo/in vitro/in situ/ex vivo nanovesicle detection. Pronanosome green fluorescence series allow to obtain fluorescently labelled nanovesicles (Green fluorescence) in a fast and easy way. ...
-
Manufactured by Rob Surgicalbased in SPAIN
Bitrack is the result of more than ten years of cutting-edge research in surgical robotics. Our value proposition is to improve the efficiency of today’s robots through unique technology, improved usability, and lower acquisition costs for ...
-
by STALICLAbased in SWITZERLAND
STALICLA’s precision medicine approach begins with ‘endophenotyping’-identifying the nonbehavioral physical and molecular presentations of NDDs and characterizing subgroups of patients with similar disease signatures. DEPI matches these patient subgroups with an NDD-targeted drug (or drug combination) by integrating and analyzing large omics data sets, including genomics, ...
-
Manufactured by Suvodabased in USA
Full visibility and automated control. Extend your clinical trial command and control center with Suvoda’s integrated ...
-
Manufactured by Biomedal S.L.based in SPAIN
iVYLISA GIP Stool is a quantitative Sandwich ELISA designed to detectandquantify Gluten Immunogenic Peptides (GIP) in stool samples. The capturing and detecting antibody is the G12, which is designed against the 33-mer peptide and which recognizes it and other equivalent immunotoxic peptides 14. These antibodies have shown great sensitivity and specificity when they have been used to certify ...
-
Manufactured by Histocellbased in SPAIN
ASC pre-differentiated to osteoblasts in Histobone scaffold (engineered synthetic bone substitute) for the treatment of bone defects caused by pseudoarthrosis, infections, resections, ...
-
Manufactured by Biotest (U.K.) Ltd.based in UNITED KINGDOM
Zutectra® is an anti-hepatitis B immunoglobulin for subcutaneous (s.c.) application used for prevention of hepatitis B virus (HBV) re-infection in HBsAg and HBV-DNA negative adult patients at least one week after liver transplantation for hepatitis B induced liver ...
-
Manufactured by ICU Medical, Inc.based in USA
Maintain a needlefree closed system to help minimize exposure to hazardous drugs and comply with USP. Prohibits the escape of hazardous drug or vapor concentrations, Blocks the transfer of environmental contaminants into the system, Eliminates needlestick injuries while minimizing , hazardous drug exposure. With the ChemoLock needlefree CSTD, it has never been easier to keep yourself safe from ...
-
Manufactured by Cytes Biotechnologiesbased in SPAIN
Acquisition of hepatocyte samples is now a critical step for research activities. The limited availability of human hepatocytes makes cryopreservation a good option to provide researchers with a sufficient amount of viable liver ...
-
Manufactured by Private Pharma Ltdbased in UNITED KINGDOM
STYLAGE XXL can give your skin the tightness it needs to eliminate all signs of sagging and hollowness. Using hyaluronic acid based gel sterile, formulated from an animal free and biocompatible hyaluronan serum that magically lifts even the deepest of wrinkles away from around the ...
-
Manufactured by Sartorius AGbased in GERMANY
Innovation is at the heart of everything we do. Our proprietary drug delivery technology platform, Veltis®, uses engineered recombinant albumin to enhance albumin’s intrinsic characteristics for optimal therapeutic performance of your ...
-
by CZ VACCINESbased in SPAIN
CZ Vaccines offers CDMO biotech to third companies and holds EU GMP certificate. CZ Vaccines is an ideal partner for any organization intending to advance their biological products from the development stage until clinical testing phase I and II as well as to production on a commercial scale. ...
-
Manufactured by NEOS Surgery S.L.based in SPAIN
Cranial LOOP cranial fixation devices are a smart system made of PEEK-OPTIMA™, a biocompatible polymer, for securely fixing bone flaps resulting from craniotomies. It is completely instrument-free due to its fast and easy ‘Pull and tighten’ action, which allows the surgeon to fixate a standard bone flap in less than a minute with only three devices, thus achieving optimal ...
-
Manufactured by Suvodabased in USA
Overcome the traditional challenges of electronic outcomes data collection and submission for complex clinical ...
-
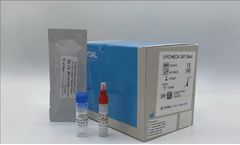
Manufactured by Biomedal S.L.based in SPAIN
iVYCHECK GIP Stool is an immunochromatographic test designed to detect Gluten Immunogenic Peptides (GIP) in stool samples. Firstly, gluten must be extracted from the sample, and then proceeding to the detection of GIP by reaction with the conjugated antibodies present in the strip. In the case of GIP in the sample due to gluten consumption, a red line will appear (positive result), while the ...
-
Manufactured by LVD Biotechbased in SPAIN
Hydrax® product range is a versatile coating system applicable to many substrates used in medical devices. Hydrax® attracts and retains water to the surface. It enhances the surface lubricity and reduces possible tissue trauma. Hydrax® technology is primarily designed to enhance interventional procedures in coronary, peripheral, and neurovascular therapies, due to excellent lubricity ...
-
Manufactured by Neuroelectricsbased in SPAIN
Mobile, comfortable, precise and robust wireless EEG. Ideal for out-of-the-lab and BCI applications. Welcome to the next generation of medical grade EEG ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you