Anatomy Articles & Analysis
-
Showcase
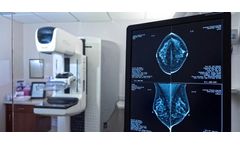
Northern Arizona, Verde Valley MC Highlights VeraForm; Importance Marking Breast Surgical Site
As a patient facing breast cancer surgery, you should know that precise "marking" of your cancer surgical site is an important part of your treatment. A marker is a device inserted at the surgical site. Precision in marking helps make sure you retain as much normal breast tissue as possible with the surgery. This ultimately leads to best overall post-surgical care by your wellness team. A new ...
-
EchoPixel’s True3D Receives Medical Device Registration in Japan
EchoPixel’s True3D software platform, a pre-operative planning technology that creates a hologram of the heart for study before an incision is ever made, has received PMDA Medical Device Registration and Approval in Japan. This first-of-its-kind software enables the entire heart surgical team to develop a precise personalized plan by interacting with specific organs and tissues as if ...
-
C4 Imaging Announces FDA 510(k) Clearance of its Non-metallic, Multimodality C4 Fiducial Marker
C4 Imaging LLC announces U.S. Food and Drug Administration 510(k) clearance of its third major product, the Multimodality C4 Fiducial Marker. This novel positive-signal MRI marker will be implanted into patients in situations where the location of specific anatomy, normal or diseased, needs to be marked for a future medical procedure. The device can be visualized using MRI, CT or x-ray, and ...
-
Lumendi Introduces EZ Glide Hydrophilic Coating for Its DiLumen Endoluminal Interventional Platform (EIP)
Connecticut-based medical device innovator Lumendi, LLC (www.lumendi.com) has launched EZ Glide, a proprietary hydrophilic coating applied to the inner sheath of its DiLumen EIP, a double-balloon endoluminal device that aims to safely improve the navigation of the endoscope through the bowel, create a stabilizing therapeutic zone inside the colon during endoluminal interventions, ...
By Lumendi Ltd.
-
FDA 510(k) Clearance
ENvizion Medical announced that the ENvue™ Electromagnetic Feeding Tube Placement System and ENvizion Feeding Tubes received FDA 510(k) clearance. This new generation of electromagnetic placement technology addresses current technological limitations and meets the growing clinical need for accurate feeding tube placement at bedside. Globally, over 22 million nasal feeding tubes are placed ...
-
Corindus Announces Global Launch of technIQ™1 Smart Procedural Automation Series for CorPath® GRX System
Corindus, A Siemens Healthineers Company and one of the leading developers of precision vascular robotics, announced today the global launch of a new set of automated robotic movements in the technIQ Series designed for the CorPath GRX System. The company received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for new software automation that provides predictable and consistent ...
-
Confidentiality Agreements and Your Medical Device Idea (Free Template)
How can you protect your idea? I regularly advise clients on the importance of protecting your new medical device ideas and how that relates to your investment in developing them. My general recommendation is protecting your idea as a 2-tiered approach. First, establishing Confidentiality with anyone you share your idea with and Second, pursuing patents. The focus of this article will be on ...
-
BIOMODEX Technology Enables New Milestone in Interventional Cardiology
BIOMODEX, the leader in bio-realistic haptic simulators for patient-specific rehearsal and physician training, today announced that its LAACS (Left Atrial Appendage Closure System) solution was used to perform a simulation ahead of a live case to be presented at the TVT Structural Heart Summit in Miami, FL. Physicians at the Montreal Heart Institute in Montreal, Quebec recently rehearsed the ...
By BIOMODEX
-
Sight Diagnostics raises $71 million amidst Covid-19 demand for blood count devices
Israeli blood testing startup S.D. Sight Diagnostics Ltd. has raised $71 million from international venture capital funds and U.S. corporations, Clal Biotechnology Industries Ltd. revealed in a report sent to the Tel Aviv Stock Exchange last week. Sight Diagnostics has yet to announce the details of the new funding round, but Clal Biotechnology Industries stated in its report that following the ...
-
SurGenTec® Announces First-of-its-Kind FDA Clearance for its 3D GraftRasp System™ Including Key Spine Indications
The 3D GraftRasp System is the only device to receive FDA clearance that allows for both decortication of bone and the delivery of autograft, allograft or synthetic bone graft. It is approved for general orthopedic use and spine procedures. “In spine procedures, the transverse processes and facet joints may now be targeted in a more efficient and effective surgical technique pioneered by ...
-
Announcement – New Urological AI Tool for 3D Reconstruction of the Ureter
Innovative technology utilizes 2D fluoroscopic images and reconstructs an accurate 3D model of the ureter for planning and guidance during ureteroplasty. RSIP Vision, a leader in driving innovation for medical imaging through advanced AI and computer vision solutions, announces today a new tool for 3D reconstruction of the ureter. This tool transforms 2D fluoroscopic images of the ureter into a ...
By RSIP Vision
-
Rapid Medical Marks First Procedures with Numen Coil from MicroPort Scientific
Rapid Medical, a leading developer of advanced neurovascular devices, announces the expansion of its portfolio in the United States and the first Numen™ coil embolization procedure. Rapid Medical now has an exclusive distribution agreement with MicroPort NeuroTech, a subsidiary of MicroPort Scientific Corporation. MircoPort’s global presence offers nearly 300 medical solutions to ...
-
How our partnership with Fast Radius is transforming patient care across North America
Fast Radius is a leading cloud manufacturing and digital supply chain company. Their Cloud Manufacturing Platform™ is a first-of-its-kind solution that integrates design, production, and fulfillment operations through a common digital infrastructure to make manufacturing easier, more accessible, and more sustainable. Founded in 2017, Fast Radius is headquartered in Chicago, IL with offices ...
By Axial3D
-
OnLume Surgical Awarded $2 Million Grant from National Cancer Institute to Advance the Development of its Novel Fluorescence-Guided Surgery Device
OnLume Inc., an early stage medical imaging company with unique technology for improving surgical precision, has been awarded a substantial grant from the U.S. National Institutes of Health (NIH)’s National Cancer Institute (NCI) to advance development of its proprietary device for Fluorescence-Guided Surgery (FGS). OnLume has developed novel FGS imaging technology to illuminate critical ...
By OnLume Inc.
-
Dr. Frank Phillips is First in the World to Use Augmented Reality Surgical Guidance in Minimally Invasive Spine Surgery
CHICAGO--(BUSINESS WIRE)--Midwest Orthopaedics at Rush announced today that Dr. Frank Phillips, Professor and Director of the Division of Spine Surgery and the Section of Minimally Invasive Spine Surgery at Rush University Medical Center, completed the first augmented reality (AR) minimally invasive spine surgery. The Augmedics xvision™ Spine System surgical guidance system allows a surgeon ...
By Augmedics
-
DT MedTech Announces Successful Total Ankle Revision Procedures Using The New Hintermann Series H2 Tibial Assembly Component and PE Inlay
DT MedTech, LLC (DTM) announced today that their new Hintermann Series H2 Tibial Assembly component and PE inlay have been successfully implanted by Prof. Beat Hintermann in three patients needing total ankle revision surgery. World-renowned foot and ankle surgeon, Prof. Hintermann, developed the new, versatile, two-component, semi-constrained total ankle replacement prosthesis, Hintermann Series ...
-
Promising Clinical Study Results of the Zenflow Spring System Featured at the AUA 2021 Meeting
Study results from the Spring System from Zenflow, Inc., a medical device company developing a minimally invasive treatment for urinary obstruction caused by enlarged prostate, or benign prostatic hyperplasia (BPH), were featured at the American Urological Association 2021 annual meeting. The Spring System was designed with the patient experience in mind and relies on a small springlike coil to ...
-
Medical Microinstruments Secures $75M to Advance Robotic Microsurgery
PISA, Italy - July 20, 2022 - Medical Microinstruments, Inc. (MMI), a robotics company dedicated to improving clinical outcomes for patients undergoing microsurgery, today announced it has raised $75 million in Series B financing. Deerfield Management led the round with participation from new investors, RA Capital Management and Biostar Capital, as well as existing investors, Andera Partners, ...
-
OnLume Surgical Participates in Clinical Trial Combining Fluorescence-Guided Surgery Imaging Device with Alume’s Novel Nerve-Targeting Agent
OnLume Inc., a Madison, Wisconsin-based medical imaging company with unique technology for improving surgical precision in the operating room, has announced the start of participation in a clinical trial combining its fluorescence-guided surgery (FGS) imaging device with a novel nerve-targeting agent. OnLume has unique FGS imaging technology to illuminate critical anatomy in real time during ...
By OnLume Inc.
-
Ultrasound Technology Answers COVID-Era Medical Training Challenges
The disruptive impact of the Covid-19 pandemic on medical services has been a central concern across our industry. The global health strategy, backed by guidance from the National Institutes of Health and World Health Organization, has so far focused on flattening the epidemiological curve. By doing so, we’ll prevent healthcare services, resources, and medical staff from being overwhelmed. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you