Neurology Equipment Supplied In California
-
based in USA
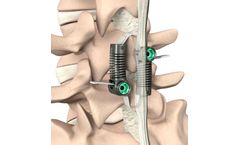
LimiFlex is intended to provide segmental stabilization following decompression for grade 1 lumbar degenerative spondylolisthesis with spinal stenosis. LimiFlex is implanted through a dorsal approach, typically through the same incision used for the surgical ...
-
Manufactured by IGeneXbased in USA
The Miscellaneous Collection Kit is compatible with lyme disease and tick-borne illness tests that require samples other than blood or urine, such as cerebral spinal fluid (CSF), tissue, placenta, breast milk, etc. We also offer a Blood Collection Kit and Urine Collection Kit that are compatible with other tests. Please note that the Miscellaneous Collection Kit is not the actual test. You still ...
-
Manufactured by Alkahest, Inc.based in USA
Another important detrimental chronokine to the biological process of aging is known as beta-2 microglobulin ...
-
Manufactured by SpineAlign Medical, Inc.based in USA
SpineAlign’s nitinol implant is a dynamic tool with unique advantages for treating painful osteoporotic vertebral compression fractures. SpineAlign offers spine specialists the ability to choose fracture-specific implants in sizes and configurations to fit each patient. The VerteLift Implant’s superior flexibility allows it to be precisely placed - even repositioned if ...
-
Manufactured by Allergan Aestheticsbased in USA
BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to temporarily improve the look of moderate to severe forehead lines, crow’s feet lines, and frown lines between the eyebrows in ...
-
based in USA
We have also developed another class of compounds that are novel histone deacetylase inhibitors (HDACi). Designated as our LB-200 series of compounds, these have potential use in the prevention and treatment of neurodegenerative diseases. The series has the potential to be the most effective in its class and may be useful for the treatment of chronic hereditary diseases, such as Gaucher’s ...
-
Manufactured by ALZET Osmotic Pumpsbased in USA
These stainless steel devices facilitate stereotaxic placement of brain cannula onto the skull of laboratory animals for CNS delivery applications. They are specially designed to grip the removable tab on all ALZET brain infusion cannulae during brain cannulation. These cannula holders are compatible with most of the common stereotaxic frames available (i.e., Stoelting, Kopf, WPI, MyNeuroLab and ...
-
Manufactured by Allergan Aestheticsbased in USA
Breast implants are not considered lifetime devices. The longer people have them, the greater the chances are that they will develop complications, some of which will require more surgery. Breast implants have been associated with the development of a cancer of the immune system called breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). This cancer occurs more commonly in ...
-
Manufactured by iWALKFree, Incbased in USA
iWALK crutch is a carefully engineered multiple award winning medical mobility device that ends the pain and inconvenience of conventional crutches. Easy to learn, easy to use, crutches and knee scooters are now ...
-
Manufactured by MedClinebased in USA
Reflux Relief System; 95% of MedCline users report better sleep. 1:1 access to a Sleep Specialist. CertiPUR-US®, gel-infused foam that’s built to last and for cooling comfort. Available in three sizes for optimal comfort. Patented arm pocket for flexible arm positioning. Includes the Reflux Relief Inclined Wedge, Insert Pillow, Therapeutic Body Pillow & set of removable, washable ...
-
Manufactured by MedClinebased in USA
Available in 2 sizes. 95% of MedCline users report better sleep. 1:1 access to a Sleep Specialist. CertiPUR-US®, gel-infused foam that’s built to last and for cooling comfort. Patented arm pocket for left- or right-side sleeping. Includes the Shoulder Relief Inclined Wedge, Insert Pillow, Therapeutic Body Pillow & set of removable, washable ...
-
Manufactured by ClearPoint Neuro, Inc.based in USA
The SmartFlow MR Compatible Ventricular Cannula has received 510(k) clearance from the FDA for use in the US for the aspiration of CSF, or injection of the chemotherapy drug Cytarabine into the ventricle. It has also been CE marked for use in Europe for the delivery of approved fluids into the brain or aspiration of CSF. It’s being utilized in approved clinical and preclinical studies for ...
-
Manufactured by Pulse Biosciences, Inc.based in USA
Unique non-thermal, cell-focused effect while preserving surrounding non-cellular tissue, primarily the collagen foundation of the skin. Multi-use handpiece. Range of single-patient use treatment tips to accommodate a variety of lesion sizes. Automated treatment settings based on treatment tip being utilized. ...
-
Manufactured by Neuralinkbased in USA
A web of communication that allows you to move, think, feel and ...
-
Manufactured by Inscopix Inc.based in USA
The nVoke System integrates in vivo cellular-resolution calcium imaging with simultaneous or sequential optogenetic manipulation to causally link neural circuit activity with behavior. Record cellular-resolution calcium imaging with optogenetic manipulation. Visualize and manipulate the same field of view longitudinally. Study the impact of discrete manipulation on behavior and its underlying ...
-
Manufactured by Stemedica Cell Technologies, Inc.based in USA
itMSCs (ischemia-tolerant mesenchymal stem cells) are “trophic” cells that circulate throughout the body and release proteins and growth factors into the damaged tissue, rescuing damaged cells from death and creating the right conditions for the newly mobilized cells to proliferate and repair. Stemedica’s itMSCs are extracted from donated bone marrow and then expanded using our ...
-
based in USA
The TwinFlo™ Catheter is intended for general intravascular use in the peripheral vasculature in arteries 3.5 mm and larger. Once placed in the selected region, the catheter can be used for infusion of diagnostic and/or therapeutic agents, and for controlling blood flow to the selected region when connected to an extracorporeal circuit. The diagnostic and/or therapeutic agents are to be ...
-
based in USA
The TAPAS® Catheter is intended for general intravascular use in the peripheral vasculature in arteries 1.8 mm and larger. Once placed in the selected region, the catheter can be used for infusion of diagnostic and/or therapeutic agents, and for controlling blood flow to the selected region. The diagnostic and/or therapeutic agents are to be used in accordance with specifications outlined by ...
-
Manufactured by Q`Apel Medical Inc.based in USA
I’m a balloon guide catheter specifically designed for stroke patients. Before I came along, balloon-based variable stiffness catheters weren’t used all that often, because all manner of technological constraints stood in the way. I’m here to change that. My particular specifications are unique in this space—which means I’m ideally positioned to serve stroke ...
-
Manufactured by Inari Medicalbased in USA
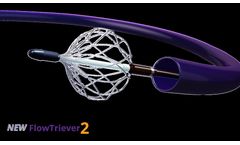
The FlowTriever is an over-the-wire system designed to: Remove clot through both mechanical and aspiration mechanisms of action, Capture and Remove large clot burden from big vessels, Treat in a single session, Eliminate the need for thrombolytics, Eliminate ICU ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you