Neurology Equipment & Supplies In Kentucky
-
PremiumManufactured by Environics, Inc.based in USA
The Environics Series Reduced Oxygen Breathing Device 3 is the newest in our line of systems that simulate altitude exposure and can be utilized for both research and hypoxia training. It is customizable for expanded research ...
-
PremiumManufactured by Environics, Inc.based in USA
The Environics Series Reduced Oxygen Breathing Device 2 is the next generation system that simulates altitude exposure and can be utilized for both research and hypoxia ...
-
PremiumManufactured by Bertin Technologiesbased in FRANCE
Bertin Bioreagent offers primary and secondary antibodies for laboratories. As essential tools, they are particularly versatile when properly ...
-
Manufactured by Integer Holdings Corporationbased in USA
Our implantable batteries are built upon unparalleled quality standards delivering safe and dependable power to your medical device. We provide standard options for Primary (Non-rechargeable) and Secondary (Rechargeable) cells, as well as customized power source solutions that are designed and manufactured to meet customer unique ...
-
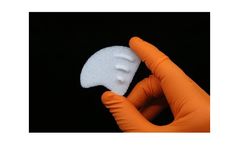
Manufactured by Anatomics Pty Ltd.based in AUSTRALIA
The StarPore® Pterional Implant has been designed to correct temporal hollowing caused by wasting of the temporalis muscle following the pterional surgical approach to the brain. The implant is supplied in standard left and right-side shapes and is easily modifiable intra-operatively. The implant has modifiable fins to allow for customisation of lateral extension. It is screwed directly onto ...
-
Manufactured by Anatomics Pty Ltd.based in AUSTRALIA
Patient specific titanium spine cages and plates can be fabricated individually from CT data to allow surgeons to restore the natural alignment of the spine and assist fusion in cases where a standard off-the-shelf implant is not suitable. These implants can be designed for either anterior or posterior fusion or vertebral corpectomy. The implants can accommodate a range of fixation screws, both ...
-
based in CANADA
The ZIP ULTRA® is Aurora Spine's minimally invasive interlaminar fixation implant for spinal fusion and was developed as an alternative to pedicle screw fixation. The ZIP ULTRA implant is designed for stabilization and load sharing in T1-S1 thoracolumbar fusion procedures, specifically for the treatment of degenerative disease, lumbar spinal stenosis, spondylolisthesis, trauma, and/or ...
-
Manufactured by CORENTEC Co., Ltd.based in SOUTH KOREA
Lospa IS system is a comprehensive stabilization solution for spinal fixation. Complex deformations and degenerative pathologies can be corrected, ensuring a successful surgical ...
-
based in USA
Minimally invasive SI joint surgery is the current medical standard of care for SI joint fusion to relieve sacroiliac joint pain. The iFuse Implant System®, available since 2009, is a minimally invasive surgical (MIS) option designed to provide immediate sacroiliac (SI) joint stabilization and allow long-term ...
-
Manufactured by Centinel Spinebased in USA
The most studied and clinically-proven total disc replacement technology in the ...
-
Manufactured by 4WEB Medicalbased in USA
4WEB Medical’s 3D printed technology utilizes engineering principles such as structural mechanics and adjacent material reaction to produce innovative spine implants that may actively participate in the healing ...
-
Manufactured by Penumbra, Inc.based in USA
The Penumbra JET 7 Reperfusion Catheter is intended for use in the revascularization of patients with acute ischemic stroke secondary to large vessel occlusion. The larger lumen of Penumbra JET 7, powered by Penumbra ENGINE®, is designed to increase thrombus removal force. The Penumbra JET 7 allows for the combined use with the 3D Revascularization Device™, which offers four ...
-
Manufactured by Spine Care Technologies, Inc.based in USA
Extentrac Elite M3D incorporates 3 Dimensional non-surgical spinal decompression. Its patented technology provides the spine care specialist with a conservative, safe, and effective means of applying customized spinal decompression combined with the synergy of evidence-based therapeutic protocols. ...
-
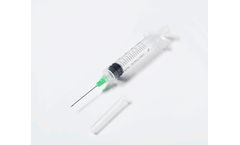
Manufactured by Numedico Technologies Pty Ltdbased in AUSTRALIA
This high quality retractable syringe is like no other on the market, and is available at a fraction of the price. ClickZip technology prevents reuse and needlestick injury due to our innovative titled needle technology that encapsulates the needle in the syringe barrel once used. This technology provides valuable protection for the health worker, their patients and others in the community and ...
-
Manufactured by Cerebro Spinebased in TURKEY
The CEREBRO Posterior Lumbar Interbody Fusion (PLIF) system is intended for use in open or minimally invasive surgery in the lumbar region and is made of radiolucent peek material.The implant is designed to be used with a sturdy and comfortable grip holder that is softened without sharp corners, allowing for controlled rotation during insertion and ...
-
Manufactured by Cerebro Spinebased in TURKEY
The CEREBRO Posterior Lumbar Interbody Fusion (PLIF) system is intended for use in open or minimally invasive surgery in the lumbar region and is made of radiolucent peek ...
-
based in USA
The Bolt 2.0 Spine Navigation System presents an advanced solution for minimally invasive spine surgery procedures. This system is designed with the surgeon in mind, focusing on simplifying and enhancing the navigation experience. It incorporates gyroscope-on-chip technology and a streamlined user interface to improve surgical accuracy and safety. The Bolt system is notable for its real-time ...
-
Manufactured by Xenco Medicalbased in USA
The CancelleX porous titanium lumbar implants are titanium foam spinal implants pre-attached to disposable, composite polymer instruments. Inspired by cancellous bone, Xenco Medical’s CancelleX porous titanium interbodies feature interconnected porosity throughout each implant. Designed to promote bone apposition and facilitate vascularization, the bio-inspired titanium foam implants ...
-
Manufactured by Techfit Digital Surgerybased in USA
Techfit custom-made cranial implants are the recommended solution for the correction of skull defects caused by trauma or oncological events, with the aim of avoiding brain atrophy and restoring aesthetics ...
-
Manufactured by Meticuly Co., Ltd.based in THAILAND
Patient-specific Cranial Mesh is a personalized cranioplasty solution designed and manufactured by professional surgeons and engineers collaborating to match the unique skull shape of each individual patient. Our personalised cranioplasty solution allows patients suffering from tumors, severe traumatic brain injuries, or severe acute strokes to regain their original skull shape back as the ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you