Medical Monitoring Equipment Supplied In Minnesota
-
Manufactured by Shockwave Medical Inc.based in USA
An effective percutaneous treatment for refractory angina, a severely debilitating condition that affects millions ...
-
based in USA
Different Platforms for Your Individual Needs.Recognizing that each surgeon’s preferences and the anatomy of each patient’s sinuses are not identical, Hemostasis has developed multiple platforms of our PosiSep® hemostat sinus splints to meet those varying needs. ...
-
Manufactured by NeuroOne Medical Technologies Corporationbased in USA
Cortical or subdural electrodes are used in electrocorticography (ECoG) or intracranial electroencephalography (iEEG) surgeries to monitor, record and stimulate the surface of the brain for up to 30 days. Our Evo cortical electrode portfolio consists of various contact configurations of strip and grid electrodes. (Rx ...
-
Manufactured by Nonin Medical Incbased in USA
SenSmart 8100S(X) Series soft sensors are designed for use in SpO2 monitoring with the SenSmart® Model X-100 Universal Oximetry System. These specially designed soft sensors offer superior patient comfort, with optimal distribution of pressure and three sizes to fit a variety of patient digits from pediatric to adult. Used with clinically proven Nonin PureSAT® pulse oximetry signal ...
-
Manufactured by Cirtec Medicalbased in USA
Long known for providing complete outsourcing solutions for Class II and III medical devices, Cirtec is pleased to announce we are now producing continuous draw Nitinol tubing. We understand that designing devices in a highly regulated industry can be challenging and our experienced and knowledgeable Nitinol team is here to help. We provide more than just Nitinol tubing, we provide vertically ...
-
Manufactured by TissXbased in USA
The development of percutaneous cardiac valves (aortic, mitral, tricuspid and pulmonary). The development of surgically implanted cardiac valves. Pericardial Patches and other tissue for surgical, vascular and other applications. Functional components for structural heart disease treatment and other vascular uses. ...
-
Manufactured by Zurich Medicalbased in USA
The innovative Zurich Blood Pressure Guidewire System Model 100 has FDA Clearance and CE ...
-
Manufactured by WR Medical Electronics Co.based in USA
The Ionto4 Iontophoresis Device delivers a reliable dose of constant current to 4 channels at once with one click, eliminating the need to pause and resume multiple stimulators. It is designed specifically for ease of use, time efficiency and current delivery accuracy during QSART testing, and is compliant with the newest safety standards. ...
-
Manufactured by Nonin Medical Incbased in USA
The NoninConnect™ 3245 finger pulse oximeter is made with the same medical-grade pulse oximetry technology used in professional Nonin pulse oximeters for patients, clinicians, and the military. You benefit from the same clinically proven technology without the need for a doctor's prescription. NoninConnect features Bluetooth® Low Energy (LE) technology for a secure, cable-free ...
-
Manufactured by United Therapeutics Corporationbased in USA
Orenitram is a prostacyclin mimetic indicated for treatment of pulmonary arterial hypertension (PAH; WHO Group 1), to delay disease progression and to improve exercise capacity. The studies that established effectiveness included predominately patients with WHO functional class II-III symptoms and etiologies of idiopathic or heritable PAH (66%) or PAH associated with connective tissue disease ...
-
Manufactured by Vascudyne, Inc.based in USA
Tissue patches are routinely used in surgery for soft tissue repair and reconstruction throughout the body in cardiovascular, orthopedic, abdominal and thoracic wall applications. Current therapies are predominantly xenogeneic, allogeneic or synthetic biomaterials such as bovine pericardium, ePTFE, small intestinal submucosa, and dermal grafts. However, issues persist: incomplete tissue ...
-
Manufactured by Ativa Medicalbased in USA
Ativa's Diagnostic Workstation with a miniaturized flow-cytometer can measure the innate immune response cells in the blood. In COVID-19, Natural-Killer Cell activation occurs within hours of first infection, these cells fight the Virus in the lungs. Miniaturized low-cost diagnostic tests Immediate lab-quality results Advanced A.I. disease screening …all at the patient ...
-
Manufactured by Skyline Medicalbased in USA
The STREAMWAY System installs in or on the wall with direct-to-drain fluid removal for safe, continuous collection and disposal.The illuminated touch screen provides safe control over surgical suction levels and displays automated measurement of volumes, while the single patient procedure filter and tissue trap both prevents cross contamination and allows for tissue retrieval. To clean, simply ...
-
Manufactured by Ilion Medical Inc.based in USA
Ilion’s surgical system features the NADIA NeuroSafe posterior bridging approach which eliminates neurovascular risks commonly associated with Laterally-Based Techniques. Our posterior approach never crosses critical neurovascular ...
-
Manufactured by Nuwellisbased in USA
The Aquadex SmartFlow System is FDA cleared to simply, safely, and precisely remove excess fluid (primarily salt and water) from patients suffering from fluid overload who have not responded to medical management, including diuretics. CLINICALLY PROVEN SOLUTION FOR TREATING FLUID OVERLOAD: Providers can specify and adjust the rate of fluid removed for each individual patient, resulting in a ...
-
Manufactured by 3M, Littmannbased in USA
Quality and reliability in one convienient package. Contains one tunable single piece diaphragm for the adult side of the stethoscope chestpiece, Black. Contains one tunable single piece diaphragm for the pediatric side of the stethoscope chestpiece, Black. Contains one non-chill bell sleeve, Black. Contains Snap Tight Soft-Sealing Eartips, Black. 3M™ Littmann® Stethoscopes offers the ...
-
Manufactured by Minnetronix Medicalbased in USA
Supporting minimally-invasive intervention in more patients, sooner. MindsEye, our proprietary expandable brain port, allows for increased visibility, safety and control to remove lesions deep in the brain. ...
-
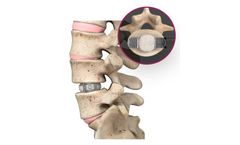
Manufactured by Spineology Inc.based in USA
The Duo Ti system represents the latest advancements in lateral interbody fusion technology. The system minimizes the amount and duration of retraction on the neural structures and surrounding soft tissues, both of which have been linked to neurologic deficits following lateral interbody fusion surgery.1, ...
-
Manufactured by Spineology Inc.based in USA
The Medius Midline Retractor System provides surgeons a low-profile retractor that allows an anatomy-conserving approach to minimize muscle disruption and retraction while providing exceptional access for direct decompression. ...
-
Manufactured by Spineology Inc.based in USA
Incite™ Cortical Fibers are a unique and versatile bone grafting option. A proprietary processing method provides thin bone fibers which expose significant osteoconductive surface area and osteoinductve endogenous growth factors. The ultrafine, entangled fibers are highly malleable to provide exceptional handling properties and support a variety of delivery options for maximum operative ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you