Medical Equipment Equipment Supplied In Minnesota
-
PremiumManufactured by Myron L Companybased in USA
The DIGITAL DIALYSATE METER is specifically designed to test both acetate and bicarbonate dialysate quality. Measure Conductivity, pH and Temperature to ensure proper mixing during dialysate preparation and as a final check of dialysate quality before hemodialysis treatment. There are no strips or reagents that expire or subjective interpretation of results. Internal sensors require the user to ...
-
Manufactured by Relievant Medsystems, Inc.based in USA
The Intracept Access Instruments are engineered for precise targeting of the BVN through a traditional unipedicular approach. The procedure is performed under fluoroscopic imaging, similar to many other minimally invasive spine procedures ...
-
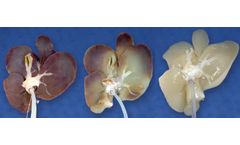
Manufactured by Reprise Biomedicalbased in USA
Our proprietary perfusion decellularization process involves cannulating the main vasculature of the organ and perfusing a mild detergent solution through the native blood vessels to result in a preserved native scaffold containing the appropriate strength and microenvironment for cellular integration after it is implanted in the patient’s ...
-
Manufactured by Zurich Medicalbased in USA
Meets the requirements of ANSI Z87.1:2015 8.1.2. for splash and droplet protection from bodily fluids; Moisture resistant polyester film; 8.5" long x 13.0" wide (21.6 cm x 33.0 cm); 0.007" thick (0.18 mm); Clear for maximum visibility; Headband and elastic not made with natural rubber latex; Able to be worn with mask and glasses/goggles; FDA registered. Made in Minnesota, ...
-
Manufactured by Thern Incbased in USA
For Ropes Between 1/4″ and 1/2″ in Diameter. The Redline Series is used to change the direction of the rope, so the winch can be mounted in a convenient location and the Redline Lead Block will control the path of the rope to avoid obstacles, reduce hazard, and improve rope ...
-
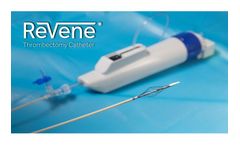
Manufactured by Surmodics, Inc.based in USA
The Vetex Medical ReVene® Thrombectomy Catheter has been engineered with a patented dynamic cage technology that enables the predictable removal of wall adherent thrombus in a single session without the need for ...
-
Manufactured by Nonin Medical Incbased in USA
The Nonin Model 3231 USB is a high-performing OEM pulse oximeter that provides SpO2 and pulse rate spot-check monitoring. A convenient USB connection allows easy data transfer to a ...
-
Manufactured by Quench Medical Inc.based in USA
Quench Medical's aerosol platform technology is designed to deliver medicine into deep areas of the lung with unprecedented efficiency to greatly increase efficacy in treating pulmonary airway diseases. ...
-
Manufactured by Shockwave Medical Inc.based in USA
Neovasc is focused on creating better outcomes for difficult-to-treat cardiology patients through the development of two novel products: Reducer™* for treatment of refractory angina and the Tiara™ System* for treatment of mitral valve regurgitation. Millions of patients worldwide with coronary artery disease who are not candidates for revascularization suffer from refractory angina ...
-
based in USA
Different Platforms for Your Individual Needs. NexPak®X Intranasal Splint is intended to prevent adhesions in the nasal and sinus cavity after surgery or trauma while minimizing bleeding and edema. NexPak®X Intranasal Splint is indicated as an intranasal splint intended to minimize bleeding and edema and to prevent adhesions between the septum and the nasal cavity. It is place in the ...
-
based in ITALY
Newronika Quality Management System ensures proper implementation and effective monitoring of design, development, purchasing, production, marketing, customer communication, and assistance processes. The implementation and maintenance of the Quality Management System represents the way Newronika has worked since the company started. The application of the Quality Management System ensures that ...
-
Manufactured by Exceleron Medical, Inc.based in USA
UltraFlow takes advantage of the latest glass nanofiber technology and proprietary binders to offer the lowest flow resistance HEPA and ULPA glass filtration media on the market. Years of research have led to the development of ultra high efficiencies along with low pressure drops for the most demanding applications. Lower flow resistance means less wear on equipment such as air compressors for ...
-
Manufactured by Biomericsbased in USA
We know balloons: creating more than 20,000 medical balloons each week. We partner with customers to design, develop, and engineer custom balloons and balloon catheters. Our scalable, end-to-end services support your project from prototype through full-scale production, packaging and distribution. Our vertically integrated structure—combined with our industry-leading expertise in polymers ...
-
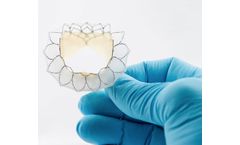
Manufactured by Anteris Technologies Limitedbased in AUSTRALIA
Evidence of Long-Term Durability: 25 patients were followed for up to 10 years; No graft failure, thromboembolic events, infections or device-related reinterventions; Echocardiographic assessment demonstrated the absence of calcification in all ...
-
Manufactured by Nextern Incbased in USA
We specialize in the design, development, and manufacturing of complex, highly specialized catheters, steerable devices, and delivery systems for interventional markets. We’ve worked with partners on steerable balloon catheters, steerable neurovascular devices, and delivery devices for structural heart implants. As your vertically-integrated partner, we have the end-to-end services to guide ...
-
Manufactured by Spectrum Plastics Group, A DuPont Businessbased in USA
We can extrude tubing with high-performance materials, thin walls, tight tolerances, and complex geometries, whether you’re looking for quick-turn prototypes or full-scale ...
-
Manufactured by INOVIQ Ltdbased in AUSTRALIA
The hTERT ICC assay is an immunocytochemistry test that is used as an adjunct to urine ...
-
Manufactured by Spineology Inc.based in USA
The Elite™ Expandable Interbody Fusion System represents the next generation of expandable technology. Its unique design minimizes both neural retraction and insertion force, accommodates a larger graft channel than many competitive expandable cages, and provides controlled expansion to restore disc height, potentially providing indirect decompression. When paired with Incite Cortical ...
-
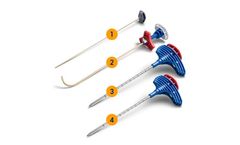
Manufactured by Spineology Inc.based in USA
Minimally invasive, bone-sparing surgical methods and large load-bearing interbody fusion systems have dramatically reduced the biomechanical demands placed on fixation systems, eliminating the need for large complex spinal fixation constructs for many patients. The Capture Facet Fixation System is the perfect complement. ...
-
Manufactured by Spineology Inc.based in USA
The VIA Spinous Process Fixation System is a next-generation system, utilizing contoured surfaces and independent compression to accommodate varying spinal anatomy. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you