Symberix, Inc.
Symberix, an early-stage spin-out of the University of North Carolina at Chapel Hill, aims to improve human health by pioneering the development of symbiotic drugs that work by controlling bacteria without killing them. Symberix’s scientific founder, Matthew Redinbo, PhD, was the first to demonstrate the feasibility of therapeutically controlling the mammalian microbiome with small-molecule symbiotic drugs. Based on this breakthrough research, the company is advancing a first-in-class approach to improve the treatment of serious lower gastrointestinal (GI) diseases as well as mitigate the debilitating lower GI side effects associated with various cancer, pain and immunosuppressive therapies. Our vision: Safe and effective pharmaceutical control of the microbiome.
Company details
Find locations served, office locations
- Business Type:
- Manufacturer
- Industry Type:
- Pharmaceuticals
- Market Focus:
- Nationally (across the country)
Product and Technology
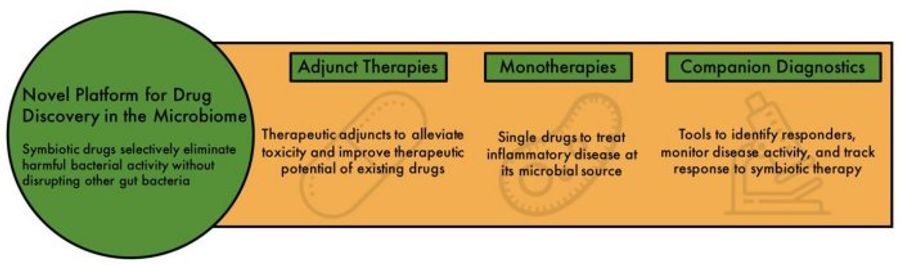
The healthy gut microbiota is composed of a diverse and balanced population of trillions of microbes whose collective actions contribute to our well-being. Gut dysbiosis—an imbalance in the composition of good vs bad microbes in the lower GI tract—contributes to a variety of human ailments and diseases. Symberix’s initial microbiome target is the beta-glucuronidase enzyme (GUS) expressed in E. coli. Preclinical studies conducted by Dr. Redinbo and Symberix show that small-molecule inhibitors of E. coli GUS ameliorated serious chemotherapy-induced lower GI side effects; protected the small intestine from NSAID-induced ulceration and leakage; and reduced anastomotic leak associated with NSAID use following GI surgery. The human gut microbiome contains a platform of nearly 300 distinct GUS enzymes. Symberix is developing novel GUS inhibitors as adjunctive therapy to improve the therapeutic window of existing FDA-approved drugs, or as monotherapy for various serious lower GI disorders. Symberix has two libraries of novel, potent, and bacteria-selective GUS inhibitors with potential NCE patent coverage as well as various broad method-of-use patent claims. The company also has access to other platforms of druggable microbiome targets and has developed broadly useful tools to facilitate the discovery of new symbiotic drugs. We anticipate initiating IND-enabling preclinical studies of a lead compound in 2020 and clinical development the following year.