Clinical Medicine Equipment Supplied In Texas
-
PremiumManufactured by Bertin Technologiesbased in FRANCE
Bertin Bioreagent offers primary and secondary antibodies for laboratories. As essential tools, they are particularly versatile when properly ...
-
PremiumManufactured by Environics, Inc.based in USA
The Environics Series Reduced Oxygen Breathing Device 2 is the next generation system that simulates altitude exposure and can be utilized for both research and hypoxia ...
-
PremiumManufactured by Environics, Inc.based in USA
The ROBD Simulator Accessory Package will simplify the set-up of the ROBD into your flight training/simulating program. The accessory package was designed to guarantee proper integration of all components with the ROBD2 or ROBD3 system (full list of included components below). Step by step instructions are provided for connecting the power, audio and video sources, for the SUT, the instructor ...
-
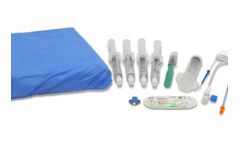
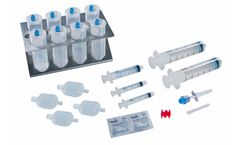
Manufactured by Prytime Medical Devices, Inc.based in USA
The REBOA Convenience Set is compatible with all Prytime Medical™ REBOA catheters and provides the clinician with the components needed for common femoral artery access, REBOA placement, and external fixation of the ...
-
Manufactured by StemBioSys, Inc.based in USA
CELLvo™ Matrix is the only commercially available substrate that aims to recapitulate biochemical as well as structural and mechanical cues of the native niche by leaving the extracellular matrix intact. Cell-derived matrices create a biologically-relevant culture environment for a variety of cell types by providing all the cues cells expect to receive in vivo in our culture dishes. This ...
-
Manufactured by StemBioSys, Inc.based in USA
CELLvo™ Matrix Plus is the next generation of CELLvo™ Matrix technology. CELLvo™ matrices are the only substrates that aim to recapitulate biochemical as well as structural and mechanical cues of the native niche by leaving the extracellular matrix intact. Cell-derived matrices create a biologically-relevant culture environment for a variety of cell types by providing ...
-
Manufactured by Thermal Angel | Estill Medical Technologies, Inc.based in USA
TA-9EXTNF, 9" IV Extension Set for quick close and ease of ...
-
Manufactured by InGeneron, Inc.based in USA
InGeneron’s Transpose® Ultra Lipoaspirate Processing System kit consists of sterile syringes, tubes, and other disposables. It is a closed system and designed to extract the regenerative cells from lipoaspirate using the Transpose® Ultra Tissue Processing ...
-
Manufactured by QinFlow Inc. (Quality in Flow)based in USA
With its unmatched performance, the Warrior is an ideal blood/IV fluid warming solution for for mid- to long-haul pre-hospitals transports, inter-facility transports, and hospital’s emergency departments that advocate simplicity and portability. Its performance makes a huge difference in environments in which aggressive bolus flows are applied in an intermittent fashion by push-pull ...
-
based in USA
Safety and peace of mind for people who live with epilepsy and for their ...
-
Manufactured by Gramercy Extremity Orthopedics, LLCbased in USA
Cannulated bone screws for reconstruction and fracture ...
-
Manufactured by Axogen (AXGN)based in USA
Axoguard Nerve Protector is the only porcine submucosa extracellular (ECM) matrix surgical implant used to protect injured nerves and to reinforce nerve reconstruction while preventing soft tissue attachments. Designed to protect and isolate, the Axoguard multi-laminar ECM separates and protects the nerve from the surrounding tissues during the healing process. The patient’s own cells ...
-
Manufactured by Seisa Medicalbased in USA
Providing in-house design, prototyping and volume production of extruded components, Seisa delivers medically compliant extrusions for the most demanding applications. We support a variety of requirements and materials, enabling end-to-end solutions for devices, ranging from catheter-based components to infusion sets and other medical ...
-
Manufactured by Avazzia, Inc.based in USA
The BEST-RSI™ device offers stronger, output microcurrent, electro-stimulation for deeper penetrating, faster, longer lasting pain relief for more serious acute or chronic ...
-
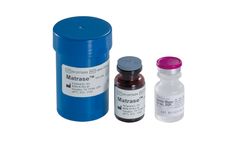
Manufactured by InGeneron, Inc.based in USA
InGeneron’s proprietary MatraseTM Reagent is a high purity enzymatic mixture for the release of mesenchymal stem cells and other regenerative cells from adipose tissue. The product is manufactured under a contract by a major biopharmaceutical company under current Good Manufacturing Practices (cGMP) and tested by high-performance liquid chromatography. MatraseTM Reagent is free of mammalian ...
-
Manufactured by Avazzia, Inc.based in USA
The BEST-PRO 1™ device offers advanced, output microcurrent with REACTION READINGS, D and Zero, electro-stimulation for more serious acute or chronic conditions where the readings make a big difference. ...
-
Manufactured by Guide Valve Limitedbased in CANADA
Cryogenic Ball Valves Design: API 6D & CSA Z245.15-17. Sizes: 2” – 36” Full & Reduced Port. Materials: Forged Stainless Steel. Pressures: ANSI Class 150 – ...
-
Manufactured by Respiratory Research, Inc.based in USA
Exhaled Breath Condensate Collection from Intubated and Mechanically-Ventilated Patients in the ICU. The RTubeVENT™ breath condensate collection device is designed for ease of use in the ICU. It can be placed in-line in the expiratory limb of the breathing circuit or at the ventilator exhaust port. This non-invasive handheld device is fully self-contained and disposable. As the subject is ...
-
Manufactured by Life Warmerbased in USA
Give warmed blood and fluids at the right time with the Quantum. Designed by medics, the Quantum was made to go far forward to the point of injury in order to start transfusions at first contact with the patient. Minutes matter when initiating blood and fluid resuscitation, and treatment at the earliest point has shown to significantly decrease mortality, which now with the Quantum ...
-
Manufactured by Bio Molecular Systemsbased in AUSTRALIA
The world’s first magnetic induction cycler is now a registered medical device with CE-IVD and TGA approval. Mic IVD is manufactured under an ISO 13485:2016 Quality Management System. ...
Need help finding the right suppliers? Try XPRT Sourcing. Let the XPRTs do the work for you