IDE Study - i-FACTOR Superior to Autograft in Overall Success - Medical / Health Care
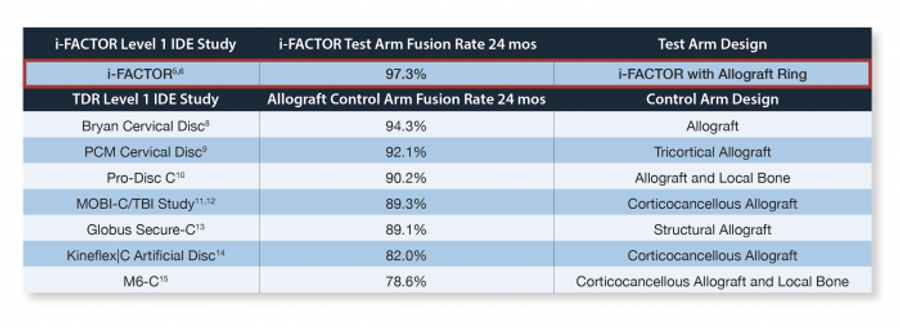
The pinnacle of scientific evidence is the Level I study. i-FACTOR Bone Graft is one of a small group of bone grafting technologies that is supported by Level I evidence. i-FACTOR Bone Graft was evaluated in a 319-patient, prospective, randomized, controlled, multi-center clinical trial assessing its safety and efficacy compared to standard-of-care (autograft). Patients underwent anterior cervical discectomy and fusion (ACDF) and received either i-FACTOR Bone Graft or local autograft in a cortical allograft ring implanted into the target vertebral space prior to placement of the screw/plate fixation construct. The results of this study have been published in the peer-reviewed journal Spine.
-
Most popular related searches
Overview Applications & Industries Served
Primary Endpoints
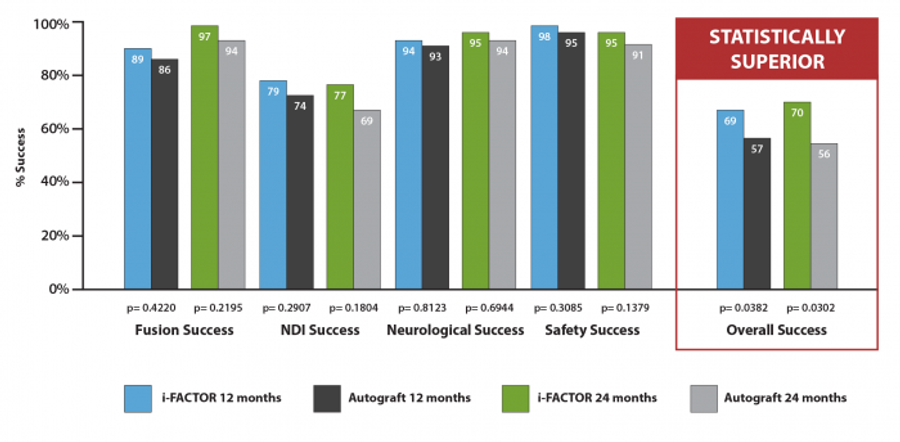
i-FACTOR Bone Graft met all four pre-specified primary endpoints investigated in this study by demonstrating non-inferiority to autograft relative to fusion rate, improvement in neck disability index and neurological success. Additionally, there was no statistical difference between i-FACTOR Bone Graft and autograft relative to the rate of adverse events.
Overall Success
An assessment of “overall success”, as judged by success in all primary endpoints, was applied to the data analysis in this investigation. The i-FACTOR Bone Graft group demonstrated 68.75% overall success. The autograft control group demonstrated 56.94% overall success. The overall success was a statistically significant difference favoring the i-FACTOR Putty investigational cohort (p=0.0382).
Efficacy of i-FACTOR Bone Graft versus Autograft i...
Efficacy of i-FACTOR Bone Graft versus Autograft in Anterior Cervical Discectomy and fusion
Results of the Prospective, Randomised, Single-blinded Food and Drug Administration Investigational Device Exemption Study
Paul M. Arnold, MD, Rick C. Sasso, MD, Michael E. Janssen, DO, Michael G. Fehlings, MD, PhD, Joseph D. Smucker, MD, Alexander R. vaccaro, MD, PhD, Robert F. Heary, MD, Ashvin I. Patel, MD, Benoit goulet, MD, Iain H. Kalfas, MD, and Branko Kopjar, MD, PhD Spine, 2016;41(13)
Background: The objective of this study was to investigate efficacy and safety of i-FACTOR Bone Graft (i-FACTOR) compared with local autograft in single-level anterior cervical discectomy and fusion (ACDF) for cervical radiculopathy.
Methods: Patients randomly received either autograft (N=154) of i-FACTOR (N=165) in a cortical ring allograft. Study success was defined by non-inferiority in fusion, Neck Disability Index (NDI), and Neurological Success endpoints, and similar adverse events profile at 12 months.
Conclusions: i-FACTOR has met all four FDA mandate...
Conclusions: i-FACTOR has met all four FDA mandated non-inferiority success criteria and has demonstrated safety and efficacy in single-level ACDF for cervical radiculopathy. i-FACTOR and autograft groups demonstrated significant post-surgical improvement and high fusion rates.