Cell Line Development Workflow - Medical / Health Care
The new standard for quality, capacity, and speed. Deliver the best cell lines. Set your IND up for success by getting FDA-accepted monoclonality assurance in just days.
-
Most popular related searches
Screen thousands of clones in parallel and select ...
Screen thousands of clones in parallel and select top clones for even non-traditional antibody molecules, like bi-specifics.
Opto™ Assure minimizes risk of costly late s...
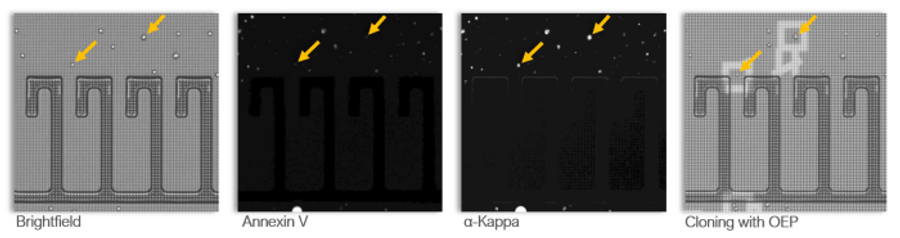
Opto™ Assure minimizes risk of costly late stage failures by identifying clones early on that secrete product with high yield and desired quality. Opto Assure assay for aggregation enables direct detection of product aggregates within days of single cell cloning. This lets you confidently identify and select clones that secrete high-quality complex molecules and develop better production cell lines faster.
>99% MONOCLONALITY ASSURANCE
>99% MONOCLONALITY ASSURANCE
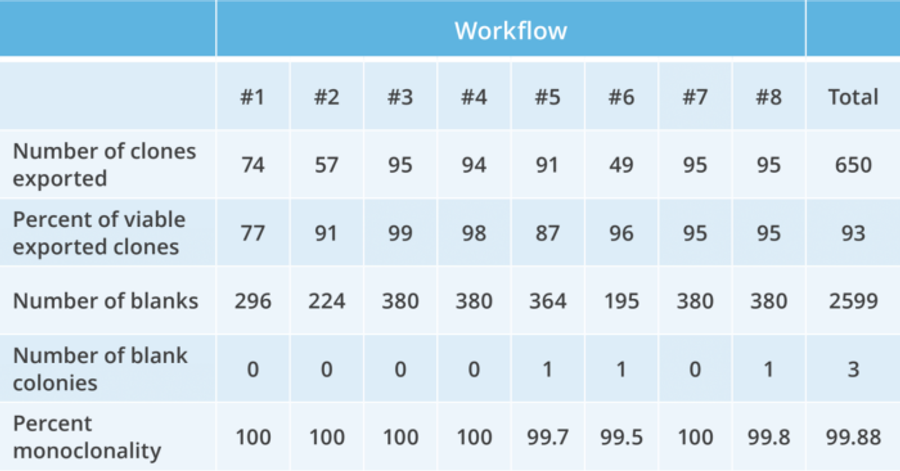
Get >99% monoclonality assurance with a single, automated round of cloning.
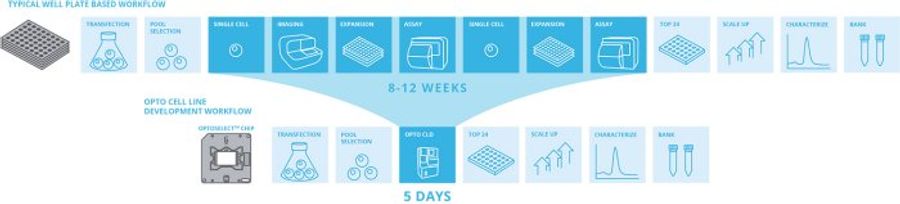
The Berkeley Lights Opto CLD workflow enables in-line controls to measure clonality during clone recovery. After recovery of each clone, media is flushed into “blank” wells to detect any residual cells. Expand >90% of selected clones from the Beacon system when recovered into 96-well plates, with >99% monoclonality assurance – which is equivalent to four rounds of limiting dilution!
Selective Cell Cloning with Opto CLD lets you scre...
Selective Cell Cloning with Opto CLD lets you screen up to 100,000 cells in a single 5-day workflow, enabling access to more relevant cell diversity than conventional methods for cell line development. Gently enrich transfected pools by identifying and cloning only cells that are viable and express your protein of interest. With Selective Cell Cloning, you can eliminate weeks of mini-pool processing by enabling deeper screening of bulk stable pools.
CONFIDENT CLONALITY ASSURANCE
FDA-accepted clonality assurance for a successful IND
The Beacon system’s optofluidic chip technology and integrated imaging provide direct evidence of >99% monoclonality, without manual effort and uncertainty of traditional methods. Learn how the Opto CLD workflow provides superior cloning technology and in-process quality controls for definitive evidence of monoclonality to support regulatory approvals.
EXPORT THE BEST FROM 1000S OF CLONES
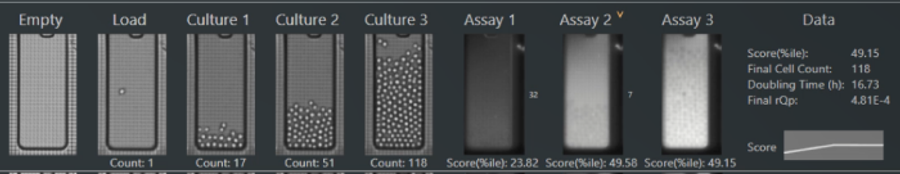
The Opto CLD Workflow enables selection of top clones by measuring growth over multiple days of on-chip culture and secretion titers using quantitative assays for both traditional and non-traditional antibodies.
- Measure antibody production from 1000s of clones
- Quantify secretion titers and specific (per-cell) productivity using the Spotlight™ Human Fc and SpotLight™ Human Kappa reagents
- Enrich and recover top-secreting clones for scale-up
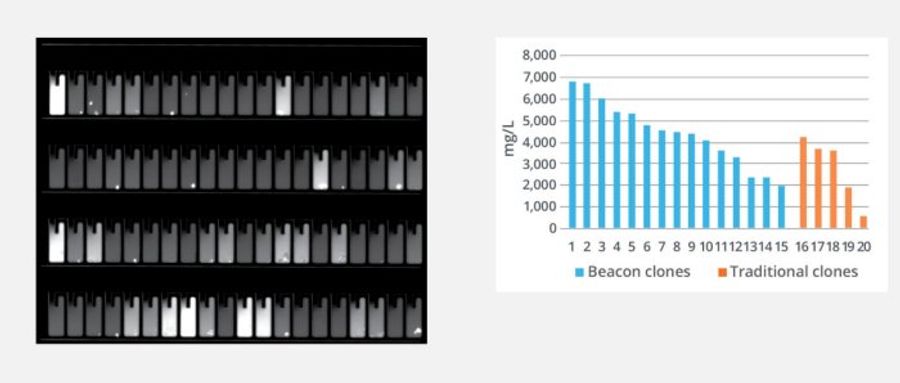
Spotlight assay: Simultaneous, quantitative antibo...
Spotlight assay: Simultaneous, quantitative antibody titer measurements on thousands of clones (LEFT). Clones selected based on titer measurements on the Beacon system had 1.5–3-fold higher titers than clones selected using traditional clone picking technology when scaled up to shake flask fed-batch cultures (RIGHT). ©2019 Catalent, Inc. All rights reserved.